Under normal circumstances, negligible amounts of blood iron enter the brain. Dr. Youdim reports that exposure to typical neuroleptics results in increased iron transport and iron deposition in brain.
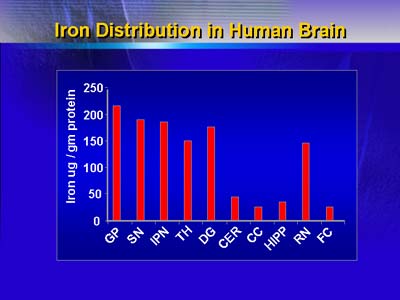
Distribution of iron in various regions of human brain. Identical results have been reported for the monkey, dog and rat brain.
Dr. Youdim simultaneously administered chlorpromazine and iron to rats and thereafter observed increased brain iron transport and significant iron deposition in cortical neurons, globus pallidus, and substantia nigra. Increased iron transport and brain iron deposition did not increase in animals treated with clozapine.
Similarly, Dr. Youdim found significant increases in brain iron concentrations in monkeys treated with haloperidol; increases in brain iron concentrations were positively correlated with dosage and duration of treatment with haloperidol.
Chlorpromazine or haloperidol treatment depleted iron in rat liver. In contrast, increased hepatic iron deposition was observed after treatment with clozapine.
Dr. Youdim believes that typical neuroleptics may bind to hepatic iron and give rise to a highly lipophilic neuroleptic-iron complex that can enter the brain and increase the permeability of neuronal membranes to iron. He speculates that brain iron deposition may result in up-regulation of dopamine receptors and degeneration of GABA-ergic neurons, eventually leading to clinical manifestations of tardive dyskinesia.
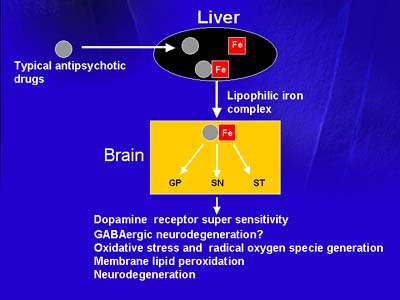
The possible mechanism by which atypical iron chelating antipsychotic drugs may transport iron (Fe) from its ferritin liver stores into the brain. In the brain, iron accumulated in globus pallidus (GB), substantia nigra (SN), and striatum (ST).
Dr. Youdim additionally speculates that pro-oxidant effects of long-term neuroleptic treatment may result in alterations of brain iron transport and iron homeostasis.
In future studies, Dr. Youdim intends to study relationships between brain iron deposition and behavioral manifestations of dopamine receptor supersensitivity in chlorpromazine-treated rats and monkeys. He believes that his findings may pertain to pathophysiology of neuroleptic-induced tardive dyskinesia in humans.