Dr. Lieberman reminded the audience that schizophrenia is a genetically based neurodevelopmental disorder with onset after puberty. When symptoms recur, there is a progressive deterioration of function. His group studied 120 patients with initial symptoms of schizophrenia who were evaluated and treated. Of these, 87% had recurrences. As the duration of initial psychosis increased, the likelihood of recurrence increased and the time to recurrence decreased. Shorter periods of psychosis predicted faster rates of treatment response. After recovery and one year of remission, 82% of patients had a second episode and 78% had a third episode. The researchers observed that recovery time lengthens with each subsequent episode.
Dr. Lieberman hypothesized that the morbidity and disability of schizophrenia is a combination of neurodevelopmental vulnerability and pathophysiology involving three processes: altered neurodevelopment, altered neuroplasticity, and progressive neurotoxicity.
(1) Altered neurodevelopment
Genetic regulation of the forebrain involves structures relevant to schizophrenia. Dr. Lieberman proposed that genetically determined dysregulation, which occurs early in development, confers vulnerability to later development of schizophrenia. He stated that a result of early dysregulation of forebrain structures is alteration of GABA (gamma aminobutyric acid) interneurons. These interneurons affect neurotransmission of dopamine and glutamate as well as that of GABA. No overt symptomatic manifestations are associated with altered neurodevelopment itself.
(2) Altered neuroplasticity
Earlier studies have demonstrated that dopamine is associated with psychotic symptoms. Excess dopaminergic activity can result in progressive degeneration by mechanisms that include behavioral sensitization to dopamine from increasing exposure of post-synaptic neurons, generation of oxidative stress disrupting neural membranes, or both. This degeneration may underlie the progressive process seen in schizophrenia.
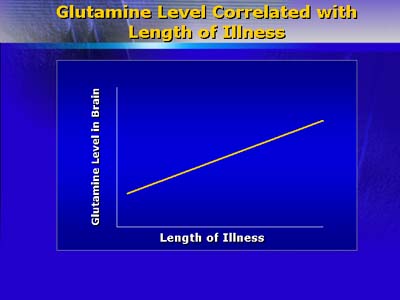
Dr. Lieberman believes that glutamate hyperfunction may be important for understanding alteration of brain function in schizophrenia. Glutamate is neurotoxic, and effects on the neurotransmitter N-methyl-D-aspartate (NMDA), which modulates glutamatergic neurotransmission, has been reported to induce schizophrenia-like symptoms. Studies have found glutamate concentration directly correlated with duration of illness in schizophrenic patients. Thus, altered neurotoxicity is modulated by the neuroplasticity of the neurotransmitters dopamine and glutamate.
(3) Neurotoxicity/neuroprogression
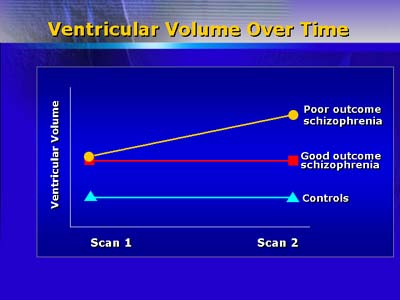
Quantitative neuroimaging (structural magnetic resonance imaging) demonstrates increased ventricular size after 4 years of schizophrenic illness. Greater ventricular volume is positively correlated with severity of psychotic symptoms. Although postmortem studies have so far not demonstrated gliosis associated with schizophrenia, Dr. Lieberman believes that gradual cell loss (apoptosis) occurs. Toxic neurotransmitter compounds can induce apoptosis, which is modulated by proteins with permissive or protective effects. Apoptosis can result in loss of cell processes (axons and dendrites), leading to reduced viability of neurons.
The concentration of Bcl-2, a protein that protects against apoptosis, is decreased in postmortem brain tissue of persons with schizophrenia. Dr. Lieberman then speculated that schizophrenia may be associated with reduced capacity to inhibit apoptosis initiated by glutamate or dopamine. He stated that some compounds under investigation have prevented glutamate-mediated apoptosis in animals, and thereby warrant further study as candidate drugs for treating schizophrenia.
In summary, Dr. Lieberman believes that the pathophysiology of schizophrenia may involve (1) altered neurodevelopment of forebrain structures leading to changes in GABA interneurons; (2) chemically mediated changes in neuroplasticity (with onset in adolescence) associated with dopamine and glutamate dysregulation, and (3) progressive neurotoxicity leading to apoptotic atrophy of selective neurons.