|
Dr. Egan and colleagues reported a high relative risk of cognitive impairments in unaffected siblings of patients with schizophrenia. They attribute this higher risk to effects on the prefrontal cortex by the gene coding for catechol-O-methyl transferase (COMT). They believe that cognitive impairment may be a marker for intermediate phenotypes of schizophrenia.
As with other disorders transmitted polygenically, genetic studies of schizophrenia are complicated by the likelihood of unidentified phenotypes. Intermediate phenotyping may be a way to address that problem. Intermediate phenotypes arise from expression of polygenes associated with schizophrenic symptoms or with traits not directly related to schizophrenic symptoms. Candidate intermediate phenotypes include eye tracking, cognitive function, and brain structural abnormalities identifiable with neuroimaging techniques. Patients with schizophrenia show various cognitive impairments that also may be manifested by unaffected siblings, suggesting a heritable component of schizophrenia-related cognitive dysfunction. Dr. Egan and colleagues studied whether cognitive impairment might reflect intermediate phenotypes in the genetics of schizophrenia.
Firstly, they estimated a relative risk of candidate cognitive impairments that included working memory, verbal declaration, attention, and psychomotor function. Their subjects were 189 patients, 292 unaffected siblings, and 111 controls with similar demographic characteristics. The subjects were assessed with a variety of cognitive and psychomotor performance tests that included an intelligence quotient (IQ) test, WRAT reading test, Wisconsin Card Sort Test (WCST), Wechsler Memory Scale (WMS), CVLT, continuous performance test (CPT), Trails A and B, and Verbal Fluency.
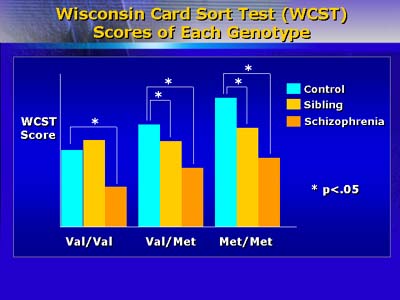
Patients performed markedly worse than controls on all tests. Siblings of patients also had significantly lower scores than did controls. Relative risk was significantly increased (p<.05) for candidate cognitive impairments by two- to four-fold.
Egan and colleagues next assessed effects of the COMT gene on cognitive function by measuring performance on the WCST, which depends upon function of the prefrontal cortex. Actions of the COMT gene include regulation of dopamine concentrations within synapses of neurons within the prefrontal cortex. The COMT gene shows a functional polymorphism whose distribution was significantly different among 175 patients, 219 siblings, and 55 controls. Moreover, differential distribution of that COMT polymorphism and performance on the WCST were significantly related. The authors believe that patterns of cognitive dysfunction may reflect intermediate phenotypes that may prove useful for studying the genetics of schizophrenia.
|