| Combination
chemotherapy with paclitaxel and platinum provides a survival
advantage in patients with relapsed ovarian cancer. Prior treatment
with taxanes did not have a detrimental effect on this survival
benefit. The benefit was the same regardless of the length of
treatment-free interval.
Currently,
there is no clear standard of care for patients with relapsed
ovarian cancer. These women are not curable. However, oncologists
would like to have a treatment that will extend overall survival
and disease free survival. One such regimen could be the combination
of paclitaxel and platinum-based chemotherapy.
Two parallel trials were undertaken to evaluate
a randomized comparison of paclitaxel-platinum combination
chemotherapy versus conventional platinum-based chemotherapy
in relapsed ovarian cancer. One trial is the International
Collaborative Ovarian Neoplasm 4 (ICON 4) Trial from centers
in the United Kingdom and Italy. The second is a smaller,
similar trial from the German group Arbeitsgemeinschaft Gynaekologische
Onkologie (AGO).
Here at ASCO, Dr. Ledermann showed the prospective
analysis of these 2 parallel trials. With a total enrollment
of 802 patients, this investigation is the largest randomized
trial ever in relapsed ovarian cancer.
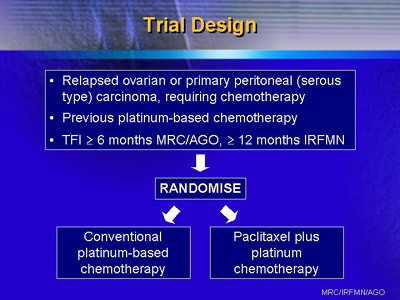
The trial was open to patients who had relapsed
ovarian cancer or primary peritoneal (serous type) carcinoma.
Entry criteria included platinum-based chemotherapy for at
least 6 cycles prior to study entry (at least 12 cycles for
the Italian group).
Investigators from centers in the U.K., Italy,
Norway, Germany and Switzerland randomized patients to conventional
platinum-based chemotherapy with carboplatin or cisplatin,
or to paclitaxel plus carboplatin or cisplatin. The regimens
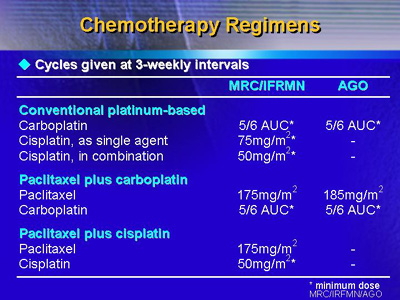
were as follows:
Pre-treatment patient characteristics were well balanced across
the 2 arms. The median age was approximately 60, and 94% had
performance scores of 0 or 1. More than 70% of the women had
a treatment-free interval of more than 12 months. About 40%
of patients had previously received platinum plus a taxane
as first-line treatment. Others received either single-agent
platinum (usually carboplatin) or another platinum-based regimen.
There was a higher incidence of neurotoxicity
in the paclitaxel-platinum arm: 20%, versus approximately
1% in the control arm. In addition an 86% incidence of alopecia
was seen in the combination arm compared with 25% in the standard
platinum-based chemotherapy arm. Interestingly, there did
appear to be a lower incidence of hematologic toxicity in
the combination arm (29% versus 46%). Other toxicities were
not noted to be significantly different between the two arms.
As of this report, investigators had followed
the patients for a median of 42 months. The results show that
the combination chemotherapy arm provides a significant survival
advantage. At two years, survival increased from 50% to 57%,
or an absolute difference in survival of 7%.
There was also a significant increase in progression-free
survival favoring the paclitaxel plus platinum arm. At 1 year,
the absolute difference in progression-free survival was 10%.
Investigators also conducted exploratory subgroup
analysis. The purpose was to determine if there were any patient
factors that had a beneficial or detrimental effect on the
survival advantage. These variables included time since completion
of last chemotherapy, previous exposure to taxanes, age and
WHO performance status.
In every case, the exploratory subgroup analysis
showed no detrimental or beneficial effect of any variable.
For example, previous treatment with a taxane did not seem
to adversely affect the benefit of paclitaxel and platinum.
Likewise, a treatment-free interval of less than 12 months
did not seem to affect survival unfavorably.
Dr. Ledermann concluded that combination chemotherapy
provides a survival and progression-free survival advantage
in patients with relapsed ovarian cancer. This effect is not
influenced by prior treatment with taxanes or treatment-free
interval of less than 12 months.
|