|
|
Summary: Promising results in a Phase I trial using STI571 to treat metastatic gastrointestinal stromal tumors has led to prompt initiation of Phase II and Phase III trials by the European Organization for Research and Treatment of Cancer (EORTC). In the Phase I trial, 25 of 36 patients responded to STI571 and only 4 of the 36 patients had progressive disease during treatment.
A dose-finding Phase I trial by the European Organization for Research and Treatment of Cancer resulted in an impressive clinical response to ST1571 among patients with gastrointestinal stromal tumors and led to an acceleration of drug testing in Phase II and III clinical trials. STI571 is a selective inhibitor of the bcr-abl tyrosine kinase in chronic myelogenous leukemia, a disease for which it has demonstrated dramatic activity in early trials. The agent is also a potent inhibitor of c-kit proto-oncogene product (KIT) and platelet-derived growth factor receptor. Gastrointestinal stromal tumors, which originate in the connective tissue of the gut, have not historically responded to treatment other than surgery. These tumors express KIT. All soft tissue sarcomas, including gastrointestinal stromal tumors, also express platelet-derived growth factor receptor. Both KIT and platelet-derived growth factor receptors produce growth signals that stimulate proliferation of malignant cells. Lead investigator Dr. Allan Van Oosterom stated that the trial initially included patients with all types of soft tissue sarcomas. The responses seen with STI571 in patients with gastrointestinal stromal tumors early in the trial, however, caused the investigators to focus on that tumor type. A total of 36 patients with gastrointestinal stromal tumors and 4 patients with other soft tissue sarcomas were enrolled in the trial and received one of four dosing schedules of STI571. Of the 36 patients with gastrointestinal stromal tumors, 25 exhibited partial or near-partial (20-25% reduction) responses. Only 4 patients with gastrointestinal stromal tumors (11%) had progressive disease. By contrast, 3 of the 4 patients with other soft tissue sarcomas had progressive disease during drug treatment. 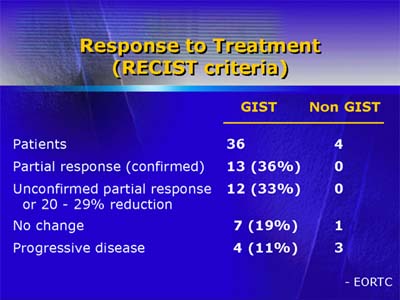
Dose-limiting toxicities at the highest dose of STI571 were observed in 5 patients, including nausea, vomiting, dyspnea, and severe leg edema. Neutropenia was also common. Intratumoral bleeding related to rapid tumor regression developed in 3 patients. 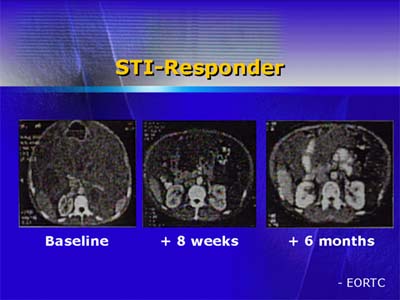
Given the results of STI571 in a disease with no previously effective treatment, Dr. van Oosterom noted, " It would be a crime not to treat a patient with a gastrointestinal stromal tumor with this drug." Phase II and III trials of STI571, including trials for small cell lung cancer, prostate cancer and glioma, are now ongoing at 15 European centers.
|
| Reporter: Jill Waalen, M.D. |

