An important medical and social problem, violent behavior has been reported to occur more frequently in persons with severe psychiatric disorders. Adding to risk in those patients is a high rate of substance abuse comorbidity. Involuntary administration of medication conveys risks of drug-related adverse effects, physical or psychological trauma, exacerbation of comorbid medical disorders, and medicolegal exposure.
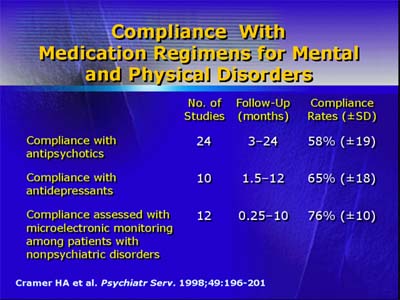
Compliance with antipsychotic medication may decrease the risk of acute, violent episodes, yet high rates of noncompliance have been well-documented in patients taking antipsychotic medications. A review of 24 studies examining compliance with antipsychotic medications showed an overall compliance rate of only 58%.
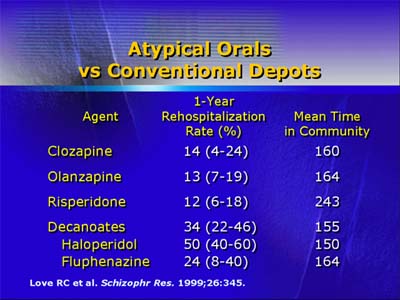
The use of long-acting formulations of antipsychotic drugs has potential to improve compliance and reduce the likelihood of acute violent episodes. Currently, two typical antipsychotic agents, haloperidol and fluphenazine, are available in long-acting (depot) formulations. In one study on rehospitalization rate, depot formulations were compared with oral formulations of various atypical antipsychotics. Results indicated that rehospitalization rates were significantly lower with orally administered atypical agents. The major limitation of that study was failure to randomize patients to different treatment groups, possibly introducing a selection bias from assigning more severely ill patients to treatment with depot formulations.
A sustained-release formulation of the atypical antipsychotic agent risperidone is now being studied in a Phase III clinical trial. Using a (Medisorb) technology that has proved successful for sustained delivery of peptides and other small molecules, the manufacturer can "set" the sustained-release duration to days, weeks, or months.
If successful, the Phase III clinical trial of long-acting risperidone may afford an effective new option for managing acutely violent patients.