Early research demonstrated that chlorpromazine induces a state
similar to hibernation in experimental animals, with lowered overall
metabolism and a shift from glycolysis to increased use of fatty
acids and ketones for energy. Dr. Dwyer believes that a triad of
antipsychotic-induced changes in humans---weight gain, hyperglycemia,
and possible ketoacidosis--- may be analogous, and reflect findings
observed in the 1950s during initial investigation of phenothiazines
as tranquilizing agents.
Using a rat neuron cell line developed for examination of glucose
uptake by neurons and characterization of changes produced by the
newer, atypical antipsychotic agents, Dr. Dwyer and his colleagues
found that antipsychotic drugs produce a dose-dependent, dopamine-independent
inhibiting effect on glucose transport.
By comparing minimal drug concentrations that inhibit glucose transport
in vitro with plasma levels observed during naturalistic treatment,
Dr. Dwyer and his colleagues found that clinically relevant clozapine
plasma levels do not inhibit glucose transport. However, plasma
levels of clozapine's active metabolites do approach threshold level
for inhibition of glucose transport, and the summed parent drug-metabolite
plasma concentration is even higher.
Comparison of the molecular shape of the transmembrane glucose
carrier GLUT 3 with chemical structures of drugs and active metabolites
is beginning to enable researchers to hypothesize about potential
drug-transport carrier interactions that may result in inhibition
of glucose uptake. That kind of analysis may explain the relatively
high affinity of clozapine for the carrier, as well as the even
higher affinity of one metabolite and the lower affinity of another.
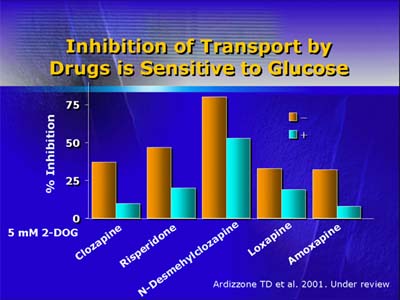
Comparing published antipsychotic drug studies with findings from
his experimental model, Dr. Dwyer found, that of all of the atypical
antipsychotics, clozapine and olanzapine were associated with the
highest rates of hyperglycemia and diabetes. Both clozapine and
olanzapine inhibited glucose uptake in his in vitro model, as did
risperidone, quetiapine, and loxapine (each associated in the clinical
literature with diabetes, but to a lesser degree than clozapine
and olanzapine).
Dr. Dwyer concluded by reminding the audience about the historical
association of antipsychotic agents and metabolic change, as well
as our growing understanding of the molecular basis for the kinds
of metabolic changes associated with antipsychotic agents. Enhanced
understanding of the molecular mechanisms mediating both antipsychotic
effects and inhibition of glucose transport may lead to development
of antipsychotic agents with lower risks of metabolic adverse effects.