| Investigators
evaluated early treatment with the angiotensin converting enzyme
(ACE) inhibitor perindopril in patients with Duchenne Muscular
dystrophy (DMD). The results suggest perindopril may delay onset
of deterioration of left ventricular ejection fraction in these
patients.
Duchenne Muscular disease (DMD) is an inherited
disorder related to a mutation in the dystrophin gene on the
X chromosome. The clinical presentation of DMD includes constant
myocardial involvement and progressive muscle weakness. Left
ventricular (LV) systolic dysfunction typically develops after
10 years. This feature results in mortality in nearly 50%
of patients.
Experimental research suggests angiotensin converting enzyme
(ACE) inhibitors would exert a beneficial effect when used
before onset of LV dysfunction. Therefore, investigators sought
to determine whether early ACE inhibitor treatment would be
protective against LV dysfunction in young DMD patients with
normal LV ejection fraction.
Dr. Becana and colleagues conducted a 2-part study in children
with genetically proven DMD and an ejection fraction greater
than 55%. The first part of this study was a multicenter,
controlled, randomized, double-blind trial of perindopril
2-4 mg/day for 3 years. In the second part, subjects continued
on open-label perindopril for an additional 24 months. Investigators
measured radionuclide LV ejection fraction at baseline and
periodically throughout the study (6 evaluations in 60 months).
No patient received any concomitant treatment.
Investigators enrolled 60 children in the first part of the
study. Of these patients, 46 entered the second phase of the
study. There were no significant differences in clinical or
demographic characteristics at the beginning of the study.
The mean age was approximately 11 years.
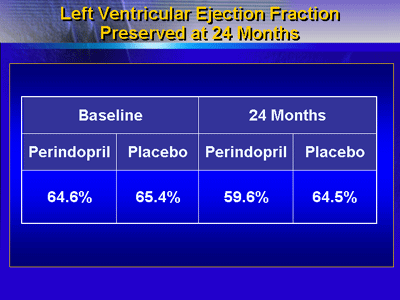
At baseline, the LV ejection fraction was 64.6% in the perindopril
group and 65.4% for placebo. At the end of the first part
of the study (24 months), LV ejection fraction was 59.6% for
perindopril and 64.5% for placebo (p = 0.114).
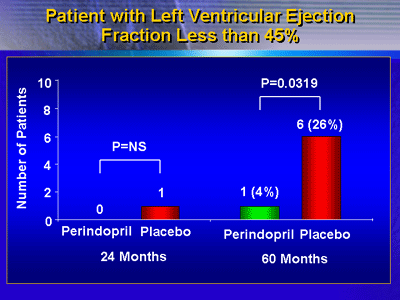
At 24 months, 1 patient in the placebo group had a LV ejection
fraction less than 45%, compared with none in the perindopril
group. However, at 60 months, 6 placebo-treated patients (26%)
had LV ejection fraction less than 45%, compared with only
1 perindopril-treated patient (4%). This difference was statistically
significant (p = 0.0319).
Investigators documented no serious adverse events in the
perindopril treatment group. In the placebo group, there were
2 patients experiencing serious adverse events (disseminated
intravascular coagulation in one, and rash/edema in the other).
There was no difference in incidence of minor adverse events
between perindopril and placebo.
These findings demonstrate that reduction in LV ejection
fraction is very common in young DMD patients. These patients
appear to tolerate perindopril treatment well. The study results
suggest early treatment with perindopril may delay the onset
of deterioration in LV ejection fraction.
|