| In children with
heart failure, carvedilol increased ejection fraction and decreased
clinical symptoms. Younger patients received the most benefit from
carvedilol.
In adults, treatment of congestive heart failure with beta-blockers
is well established. However, few data support beta-blocker use
in children. Physicians cannot extrapolate adult findings to pediatric
populations. This is because of developmental changes in myocardium,
and differences in drug metabolism and elimination.
An ongoing open study provides new data regarding the pharmacology
and clinical effect of carvedilol in children. Dr. Laer described
this study, which includes pediatric patients who were clinically
stable without further improvement of ventricular function. Ten
had dilated cardiomyopathy, and six had congestive heart failure
secondary to congenital heart disease.
All children received standard therapy including angiotensin-converting
enzyme inhibitors, diuretics, and digoxin. Investigators started
oral carvedilol with a test dose of .09 mg/kg and increased to a
target dose of .35 mg/kg twice daily. Treatment with the beta-blocker
lasted at least six months.
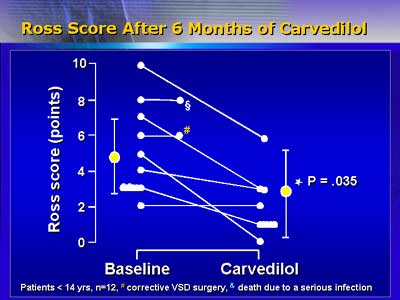
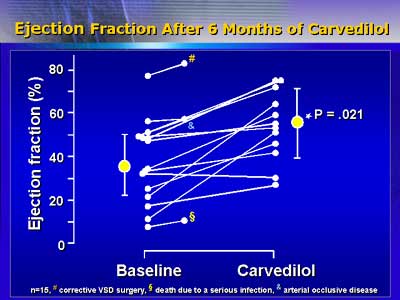
Ventricular function has improved for the 12 patients (aged 6 months
to 19 years) who have completed the study. Mean ejection fraction
increased significantly, from 37% to 52%. As clinical symptoms improved,
mean scores on a scale for severity of pediatric heart failure decreased
significantly, from approximately 5 to 3 points.
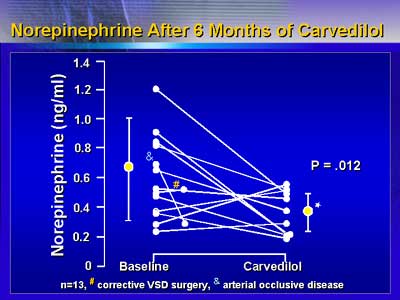
Pharmacokinetic analysis revealed an age-dependent increase in
elimination half-life of carvedilol. In addition, carvedilol concentration
at half maximal heart rate reduction was lower in the younger patients.
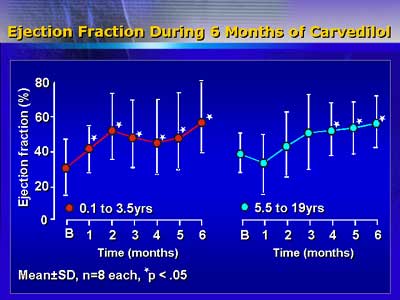
Because of these findings, Dr. Laer and colleagues now accelerate
the titration schedule from 4 to 2 weeks in younger patients. They
may try increasing the target dose in the future. This may possibly
provide more clinical benefit.
While these results are promising, the study is small and uncontrolled.
Dr. Laer said investigators should assess the benefit in larger,
randomized controlled trials. Such a study is underway right now
in the United States.
|