| The anti-arrhythmic
drug azimilide did not reduce all cause mortality in recent post-myocardial
infarction agents. However, fewer patients developed atrial fibrillation/flutter
compared with placebo.
The Azimilide Post Infarct Survival Evaluation (ALIVE) trial sought
to determine whether an anti-arrhythmic agent could reduce arrhythmic
deaths following myocardial infarction.
The trial included 3,381 patients with recent myocardial infarction
(5 to 21 days prior to study entry). All had low ejection fraction
(15% to 35%). Investigators prospectively defined a high-risk subgroup,
which they defined as depressed heart rate variability. This subgroup
accounted for 38% of patients.
All-cause mortality at 12 months was 15% in patients with depressed
heart rate variability and 9.5% in the remainder of the patients.
This confirmed that patients with depressed heart rate variability
were indeed at high risk.
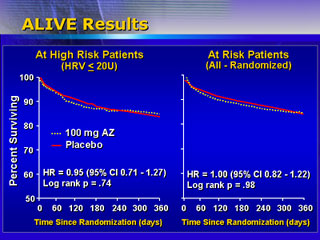
However, in this high-risk group there was no statistically significant
difference in mortality between anti-arrhythmic and placebo. There
were 642 high-risk patients on placebo and 622 on azimilide. There
were 96 and 98 deaths, respectively.
There was also no difference in mortality for all patients. Of
1,690 and 1,691 patients on placebo and azimilide, there were 196
and 197 deaths.
Another important prespecified endpoint was atrial fibrillation.
Azimilide is being developed for this indication.
Investigators looked at patients in normal sinus rhythm who developed
atrial fibrillation. There were 19 patients on placebo and eight
on azimilide. This difference was statistically significant.
They also looked at patients who started the study in atrial fibrillation.
Sinus rhythm returned in 15 of 56 patients on azimilide and only
4 of 37 patients on placebo. This finding did not quite reach statistical
significance.
There was a low incidence of torsade de pointes and neutropenia
in the azimilide arm. Non-fatal torsade de pointes occurred in five
patients on anti-arrhythmic drug and one patient on placebo. Neutropenia
occurred in 15 patients on drug versus four on placebo; none correlated
with life threatening arrhythmias, and all resolved spontaneously.
This trial did not suggest azimilide is superior to placebo in
reducing all-cause mortality. However, Dr. Camm said the results
of this trial provide further support for the development of azimilide
as a treatment for atrial flutter/fibrillation.
|