| In a randomized,
22-center trial, end-stage heart failure patients who received a wearable
left ventricular assist device had a documented survival benefit and
improved quality of life. These results demonstrate that implantable,
man-made heart pumps can prolong and enhance life in the treatment
of advanced heart failure. Implanting a left ventricular assist device
is now a viable alternative to transplant for these patients.
Left ventricular assist devices clearly benefit heart transplant
candidates as a temporary solution when a human donor is not immediately
available. These patients have enjoyed a reasonable improvement
in quality of life, with an acceptable level of complications.
However, clinicians do not know the value of left ventricular device
implantation as a part of long-term therapy for end-stage heart
failure patients.
Dr. Rose and other investigators designed a randomized evaluation
of long-term mechanical assistance for treatment of congestive heart
failure. Investigators refer to this trial as REMATCH (Randomized
Evaluation of Mechanical Assistance for the Treatment of Congestive
Heart Failure). The New England Journal of Medicine has published
an early release of REMATCH results on the web at www.nejm.org.
The REMATCH trial included 129 patients with end-stage heart failure
who were not eligible for cardiac transplantation. Investigators
randomized patients to receive either the Thoratec Vented Electric
Heartmate device or a control group of expert-guided optimal medical
management.
Dr. Rose and colleagues hypothesized a 33% reduction in death rate
over two years of observation for the group of patients who received
the left ventricular assist device. Results show instead a 48% mortality
reduction for the device group. One-year survival was 52%, versus
25% in the control group. Two-year survival was 23% versus 8%.
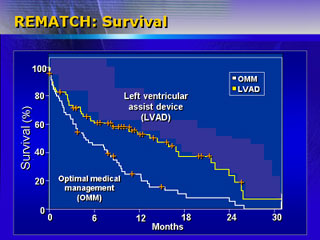
They also hypothesized that quality of life in implanted patients
would equal or exceed controls. Indeed, these patients had a significantly
improved quality of life one year after enrollment on validated
scales such as the Beck Depression Inventory and New York Heart
Association functional class.
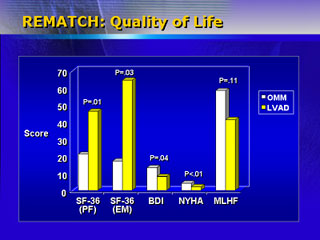
Patients who received the device did have a significantly higher
rate of complications. The frequency of serious complications was
2.35 times greater in this group. Most of the complications were
infection, bleeding or device malfunction.
Nevertheless, investigators estimate implanting 1,000 patients
with this device would save approximately 270 lives over one year.
This is about four times the effect of angiotensin-converting enzyme
inhibitors and beta-blockers. It is almost 30 times the effect of
thrombolytics in treating acute myocardial infarction.
Dr. Rose said these results proved what physicians have hoped for
decades: that an implantable, man-made heart pump device could prolong
and enhance life.
He estimated the procedure would cost US$160,000, or about the
cost of a heart transplant. However, that figure is very preliminary.
Investigators are now determining the cost-effectiveness of this
device as a treatment for advanced heart failure.
|