| In patients who
received a successful percutaneous coronary intervention, the drug
tranilast failed to reduce the frequency of major coronary events
versus placebo. However, this trial created a massive database reflecting
current clinical practice around the world. This data should be useful
to researchers who want to know more about the practice of interventional
cardiology in the modern era.
Restenosis remains a major problem after percutaneous coronary
intervention. Reported rates are as high as 50%, depending on specific
lesion and patient subset. Investigators continue to explore new
strategies that may be effective in preventing restenosis.
Dr. Holmes reported on the largest-ever interventional trial for
prevention of restenosis. This trial, which included 11,500 patients
from many different countries, is known as PRESTO (Prevention of
Restenosis with Tranilast and its Outcomes).
Preclinical models show that tranilast reduces intimal hyperplasia
and vascular smooth muscle cell migration. Small pilot studies in
humans suggest the drug is dramatically effective in reducing rate
of restenosis after balloon angioplasty, stenting and directional
atherectomy.
Based on that evidence, investigators randomized a large group
of patients to placebo or tranilast in a low or high dose, administered
for one or three months. The main requirement for study entry was
a successful percutaneous coronary intervention. About half of the
patients had unstable angina, while 25% had diabetes. Many had severe
lesions and some had chronic total occlusions.
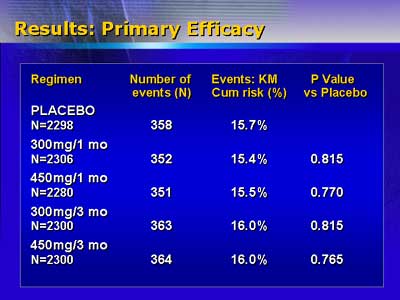
The primary endpoint of the study was incidence of major adverse
cardiac events (all-cause mortality, myocardial infarction, and
ischemia-driven target vessel revascularization) at nine months.
Investigators anticipated a 30% reduction in major adverse cardiac
events as clinically relevant. However, cumulative risk for events
was identical for the placebo group and the four tranilast groups,
ranging from 15.4% to 16%.
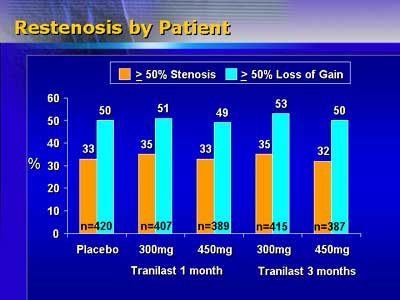
Tranilast treatment did not result in less restenosis. In an angiographic
substudy including more than 2,000 patients, the restenosis rate
ranged from 32% to 35%. Myocardial infarction occurred in 1.4% of
patients and death in 1.2% during the 9 months of the study.
While these results were disappointing, Dr. Holmes said the trial
provides researchers with an unprecedented library of data reflecting
the current practice of interventional cardiology. The sponsor of
this trial has encouraged the investigators to look at subsets of
patients as the basis for identifying new treatment strategies.
Because trial enrollment was very rapid (approximately 1,000 patients
per month), the database gives a detailed overview of the practice
of interventional cardiology over the past 1? years.
Perhaps tranilast will work better using a local drug delivery
strategy. According to Dr. Holmes, that is the subject of a Japanese
study, which will probably be conducted soon.
|