| Using
modern imaging techniques, investigators can accurately measure
the volumes of brain structures affected in Alzheimerfs disease.
Therefore, there is increasing interest in using these measurements
as outcome measures in clinical trials of neuroprotective drugs.
Dr. Doraiswamy presented results of the first randomized, controlled
trial in which investigators looked at the effect of an Alzheimerfs
disease treatment on magnetic resonance imaging markers in the
brain. Results show that donepezil (Aricept) does seem to have
an effect on these imaging markers.
The role of neuroimaging in dementia is no
longer limited to diagnosis. Investigators are now showing
that neuroimaging is useful for monitoring the progression
of brain changes, and for monitoring the efficacy of drugs
that might slow down the progression of Alzheimerfs disease.
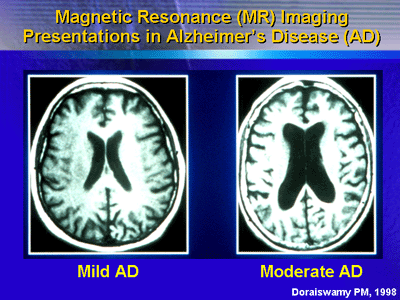
One of the earliest changes that occur in Alzheimerfs disease
is atrophy of the medial temporal lobe. Within the medial
temporal lobe are two structures: the hippocampus and the
entorhinal cortex. These structures degenerate very early
in the disease. This effect appears to be very strongly correlated
with the cognitive deficits in Alzheimerfs disease and the
future rate of decline. Therefore, measurements of these brain
structures might be useful as a surrogate marker to gauge
the degree of brain degeneration and to predict rate of decline
in patients with Alzheimerfs disease.
Modern imaging techniques allow investigators to measure
the volumes of brain structures with a high degree of accuracy
and validity. Therefore, there is increasing interest in using
these measurements as an outcome measure in clinical trials
of neuroprotective drugs. In stroke, multiple sclerosis and
other diseases, magnetic resonance imaging is already established
as an outcome measure in clinical trials.
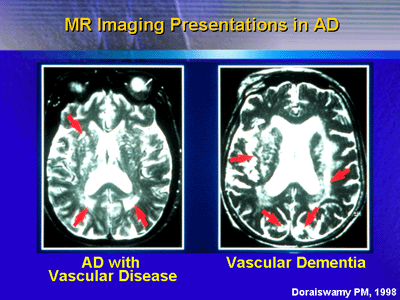
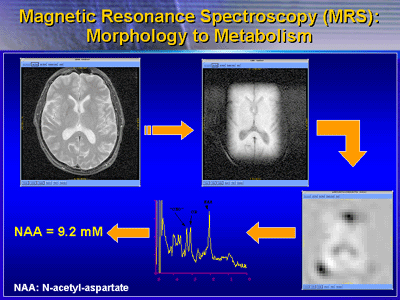
Magnetic resonance (MR) spectroscopy is one imaging technique
under development for outcome measures in Alzheimerfs clinical
trials. This imaging technique measures a brain chemical called
N-acetyl-aspartate. There is of a lot of interest in this
metabolite because it is only found inside nerve cells. Investigators
consider it as a marker of a living nerve cell. As nerve cells
die, the levels of N-acetyl-aspartate declines. Therefore,
a measurement of the amount of N-acetyl-aspartate in a particular
brain region is a good indicator of how many living nerve
cells there are in a region and how well they are functioning.
Dr. Doraiswamy presented results of the first randomized,
controlled trial in which investigators looked at the effect
of an Alzheimerfs disease treatment on magnetic resonance
imaging markers in the brain.
This trial included 77 patients with mild to moderate probably
Alzheimerfs disease. Scores on the Mini-Mental State Exam
ranged from 10 to 26. These patients received 24 weeks of
donepezil or placebo, followed by a 6-week washout period.
All patients underwent magnetic resonance imaging of the
brain at baseline and every 6 weeks during the trial. In addition,
they underwent evaluation with the cognitive subscale of the
Alzheimerfs Disease Assessment Scale (ADAS-cog) at baseline
and every 6 weeks thereafter. In addition, investigators measured
hippocampal volumes at baseline and week 24.
Data from the ADAS-cog evaluations showed that patients receiving
donepezil did substantially better than placebo at every time
point. The mean difference at the end of 24 weeks was approximately
3 points. Dr. Doraiswamy said this was similar to results
of larger randomized multicenter trials, pointing to the validity
of this smaller trial.
The imaging findings of this study suggest that donepezil
increased levels of N-acetyl-aspartate between weeks 6 and
18 in a variety of brain regions (white, periventricular,
and subcortical gray matter). However, the difference in N-acetyl-aspartate
levels versus placebo was not significant at the 24- or 30-week
evaluations.
Data on hippocampal volumes were somewhat surprising to investigators.
Some expected to see no significant differences between hippocampal
volumes in donepezil versus placebo. This was because of the
small size and short duration of the trial. However, they
found that hippocampal volumes declined by 8.2% from baseline
to 24 weeks in the placebo group. By comparison, volumes declined
by only 0.37%. This difference was statistically significant
(p < 0.01).
Findings like this could have important implications for
the development of Alzheimerfs disease treatments. Until now,
the treatment of symptoms has been the main indication for
Alzheimerfs disease drugs. If neuroimaging can conclusively
prove that drugs slow the rate of brain atrophy, the drug
may receive an indication for slowing the progression of Alzheimerfs
disease. In the future, clinical trial investigators will
increasingly use imaging markers alongside clinical markers
as outcome measures in patients with dementia.
|