|
This is the largest phase III trial of
malignant pleural mesothelioma ever conducted. The combination
of the novel antifolate drug pemetrexed and cisplatin increased
survival and improved symptoms. Because of these results, pemetrexed
plus cisplatin now should be standard front-line therapy for
patients with this disease.
Malignant pleural mesothelioma is an aggressive cancer of
the lining of the lung. The disease occurs typically 20 to
40 years after exposure to asbestos. Each year, there are
approximately 2,500 new cases in the United States and 5,000
new cases in the European Union. Experts expect incidence
of this disease to increase over the next 2 decades.
Mesothelioma survival is short, and the disease causes severe
chest pain, chronic shortness of breath, and sense of hopelessness.
There is no effective or approved chemotherapy for mesothelioma.
Pemetrexed (Alimta) has emerged as a potentially effective
drug in Phase I clinical trials.
This novel antifolate drug exhibited a 14% response rate
as a single agent in this disease. Subsequently, investigators
conducted a phase III trial of pemetrexed (500 mg/m2)
and cisplatin (75 mg/m2) versus cisplatin alone.
The primary endpoint of the phase III trial was survival.
Secondary endpoints included response rate, quality of life
and lung function. 452 patients were enrolled, of which 448
had eligibility for the final analysis.
In this ASCO presentation, Dr. Vogelzang reported a 25%
increase in survival with pemetrexed and cisplatin. (P = 0.02).
Patients on the combination had a survival of approximately
one year, versus nine months for the cisplatin arm.
This is the first time investigators have ever documented
an improvement in mesothelioma survival.
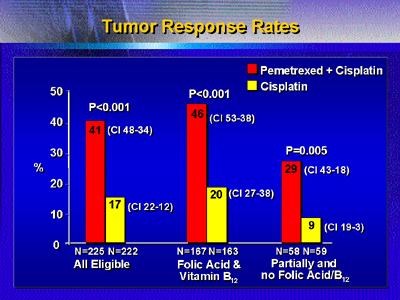
The tumor response rate was 41% for pemetrexed-cisplatin
treatment versus 17% for cisplatin. Median time to disease
progression was 5.7 months versus 3.9 months in the cisplatin
arm.
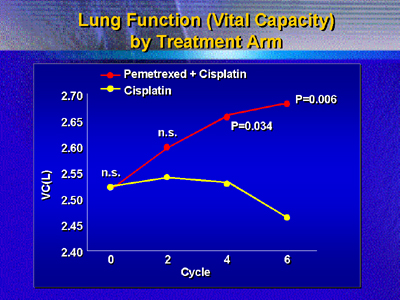
Another important factor was symptom improvement. Two independent
measures of lung function showed improvement. For example,
vital capacity gradually improved on the experimental treatment,
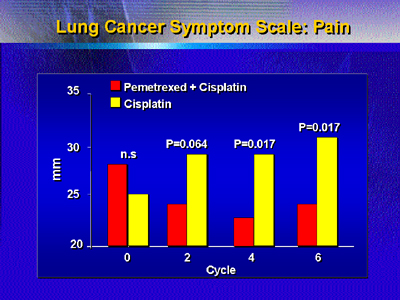
while it declined on the comparison arm. Pain on a symptom
scale for lung cancer improved greatly with pemetrexed therapy,
and worsened on cisplatin alone.
During the trial, investigators noted a high rate of toxicity
in the pemetrexed-cisplatin arm. This was linked to elevated
homocysteine. Subsequently enrolled patients received folic
acid and vitamin B12 in addition to the experimental
treatment. For patients who received this simple vitamin supplementation
regimen, toxicity and drug-related death rate decreased and
were virtually identical in both treatment arms.
|