| While
novel treatment strategies for colorectal cancer command attention,
investigators continue to gain insight into the usefulness of
cytotoxic chemotherapy for advanced disease. For example, investigators
reported at ASCO that treatment of advanced colorectal cancer
with a new drug combination containing oxaliplatin improves
average patient survival and has fewer side effects than the
previous "best" regimen of irinotecan plus 5-fluorouracil/leucovorin.
A separate analysis shows irinotecan regimen is not associated
with increased risk of early death.
Oxaliplatin Regimen: New Option
Colorectal cancer remains the second most common cause of
cancer mortality in the United States. Once the disease has
spread to distant organs, mean survival in patients who choose
not to undergo chemotherapy is approximately 6 months. Currently,
the best chemotherapeutic regimen is the combination of irinotecan,
5-fluorouracil and leucovorin. This regimen has improved average
survival to more than 14 months.
At ASCO, investigators reported a trial comparing this standard
with oxaliplatin plus 5-fluorouracil/leucovorin. Investigators
at 150 or medical centers around North America randomized
nearly 800 patients with advanced colorectal cancer into the
trial, known as N9741.
A significant survival advantage emerged for oxaliplatin
plus 5-fluorouracil/leucovorin combination. Patients on that
regimen survived an average of 18.6 months, versus 14.1 months
for patients in the standard arm of irinotecan and 5-fluorouracil/leucovorin.
In addition, time to tumor progression favored the oxaliplatin
combination by 1.9 months.
|
FOLFOX: Active and Tolerable
| - |
Irinotecan + 5-FU/LV (IFL) |
5-FU/LV + Oxaliplatin
(FOLFOX) | | Overall
survival | 14.1
months | 18.6
months | | Time
to progression | 6.9
months | 8.8
months | | Response
rate | 29
% | 38
% |
|
According to Dr. Goldberg, 18.6 months is the longest average
survival ever reported in a major trial in advanced colorectal
cancer in the United States.
Patients on oxaliplatin plus 5-fluorouracil/leucovorin had
less diarrhea, nausea, vomiting, severe infections and hair
loss than patients on the reference arm. The oxaliplatin regimen
does commonly cause numbness and tingling that is aggravated
by cold exposure. This affected many patients, but generally
only responders, since this adverse effect typically occurs
late in therapy.
These results suggest oxaliplatin deserves a place in the
armamentarium of agents for treatment of colorectal cancer,
according to Dr. Goldberg.
Prominent colorectal cancer researcher Dr. Leonard Saltz
said the regimen of oxaliplatin, 5-fluorouracil and leucovorin
now represents an additional treatment option with a distinctly
different side effect profile that may be more acceptable
to some patients. However, he cautioned against overestimating
the additional benefit this new treatment confers. Colorectal
cancer remains an important cause of cancer related mortality.
No Excess Death with Irinotecan Regimens
The irinotecan, 5-fluorouracil and leucovorin combination,
given as bolus or infusion, improves survival over 5-fluorouracil/leucovorin
alone. However, investigators recently noted a 4.5% rate of
death from any cause within 60 days of starting bolus treatment
in the N9741 study. This contrasts with previously reported
mortality rates of 1% for both the irinotecan plus 5-fluorouracil/leucovorin
regimen and 5-fluorouracil/leucovorin alone.
However, earlier trials measured mortality differently: they
typically looked at drug related deaths within 30 days of
the end of therapy.
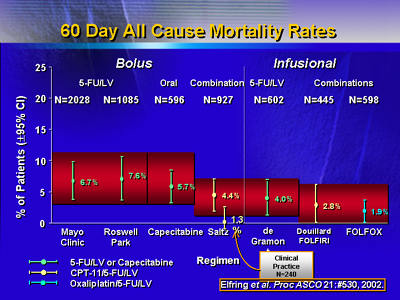
To put the 4.5% death rate into perspective, investigators
calculated 60 day all cause mortality for randomized United
States and European trials of irinotecan plus 5-fluorouracil/leucovorin
or 5-fluorouracil/leucovorin. In both registration and post
approval studies, 60 day all cause mortality rates for irinotecan
plus 5-fluorouracil/leucovorin were as similar or lower than
those for 5-fluorouracil/leucovorin.
For bolus treatment, 60 day mortality rates were 6.7% and
7.6% for the Mayo Clinic and Roswell Park 5-fluorouracil/leucovorin
regimens, respectively. Combination irinotecan plus 5-fluorouracil/leucovorin,
was actually somewhat lower at 4.4%, and lower still for oxaliplatin
with 5-fluorouracil/leucovorin (1.9%).
Investigators said the analysis showed that irinotecan-containing
regimens are not associated with excess risk of mortality,
and treatment choice should be made according to efficacy
and acute or chronic safety profiles, not concerns regarding
early mortality.
Second Line Irinotecan: Safety Differences Emerge
After first line 5-fluorouracil treatment, an approved option
for second line therapy of metastatic colorectal cancer in
the United States is irinotecan in one of two schedules. These
approved regimens, however, have never been directly compared
in a safety and efficacy trial.
Dr. Fuchs and colleagues randomized 291 patients with proven
metastatic colorectal cancer who progressed on first line
5-fluorouracil to an irinotecan regimen. The irinotecan regimens
included either a 6 week course (125 mg/m2 weekly
for 4 weeks, 2 weeks rest) or a 3 week course (350 mg/m2
every 3 weeks).
One year survival was 46% for the weekly group, and not significantly
different for the every three weeks group (41%). Median survival
was 9.9 months for both groups. Time to progression was not
significantly different (4 months for weekly and 3 months
for every 3 weeks).
Significant differences emerged in adverse effects. Grade
3-4 diarrhea occurred in 36% of the patients randomized to
weekly irinotecan, but only 19% for patients in the every
3 weeks group (P = 0.002). Also, at week 4, more patients
were able to receive full dose in the every 3 weeks group.
Yet there were significantly more cholinergic symptoms at
the first infusion for patients in the every 3 weeks group
(61% versus 31%, P < 0.0001).
|