|
A clinical evaluation of patients with
untreated advanced pancreatic cancer showed that the combination
of gemcitabine and the antifolate agent pemetrexed has clinical
activity. The regimen achieved response rates and survival that
compare favorably with results achieved with conventional therapy.
The combination of pemetrexed and gemcitabine resulted in a
high incidence of hematologic toxicity but a low rate of non-hematologic
toxicity. Overall, the regimen had an acceptable toxicity profile.
Currently available therapies for advanced pancreatic cancer
continue to yield poor results. Most chemotherapeutic regimens
achieve objective response rates of 5% or less. Median survival
ranges between 5 and 6 months, and 1-year survival averages
less than 20%, regardless of the therapy employed. The poor
outcomes with available therapies have led investigators to
explore new therapies that offer potential to improve response
rate and survival in patients who have advanced pancreatic
cancer.
Investigators evaluated the clinical impact of the addition
of antifolate agent pemetrexed to gemcitabine. Pemetrexed
has shown efficacy against pancreatic cancer specimens in
human tumor cloning assays. The agent also has produced evidence
of efficacy against pancreatic and colorectal cancer in limited
clinical evaluations. Pemetrexed and gemcitabine have demonstrated
synergy in vitro, and the combination of pemetrexed and gemcitabine
achieved an objective response rate of 21% in a phase I clinical
investigation of the combination.
Investigators at 9 centers enrolled 42 patients who had
untreated advanced pancreatic cancer. The treatment regimen
consisted of gemcitabine 1250 mg/m2 days 1 and
8 and pemetrexed 500 mg/m2 day 8, repeated every
21 days. The patients received a total of 213 cycles of therapy
and a median of 4 cycles. Most patients also received folic
acid and vitamin B12 supplementation after investigators
learned of data that showed supplementation substantially
reduces pemetrexed toxicity.
The regimen achieved 5 partial responses (12%) in 41patients
who could be evaluated for response. Additionally, 18 (44%)
patients had stable disease, including 3 patients who had
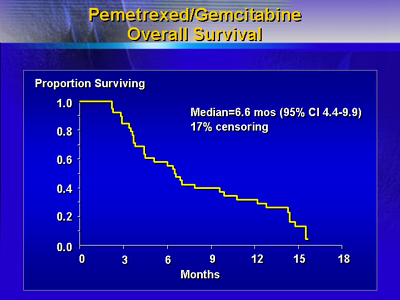
unconfirmed partial responses. The cohort had a median survival
of 6.6 months, and median time to progression was 3.1 months.
Patients had weekly evaluations for clinical benefit response,
which included performance status, pain score, analgesic use,
and weight. Analysis of clinical benefit in 30 patients revealed
a 13.3% clinical benefit response.
|
Clinical Activity of Pemetrexed-Gemcitabine
Combination Therapy
|
Parameter | -- | |
Partial Response |
5 (12%) | Stable
Disease |
18(44%) | Median
Survival | 6.6
mo | Median
TTP | 3.1
mo | 1-year
Survival | 32% | Clinical
Benefit Response | 4
(13.3%) | Time
to Response | 2
wk | Duration
of Response | 6.5
wk |
|
The combination of pemetrexed and gemcitabine caused hematologic
toxicity in most patients. Grade 3-4 neutropenia occurred
in 84% of patients, grade 3-4 leukopenia in 74%, and grade
3-4 thrombocytopenia in 33%. Relatively few patients experienced
severe non-hematologic toxicity. Grade 3 or greater non-hematologic
toxicity included diarrhea in 2%, nausea in 5%, fatigue in
4%, elevated bilirubin in 2%, elevated liver enzymes (ALT
or AST) in 24%, and elevated alkaline phosphatase in 7%.
Results of the study show that the combination of pemetrexed
and gemcitabine has clinical activity in advanced pancreatic
cancer. The data also indicate that the regimen has an acceptable
toxicity profile.
|