|
German investigators found that the combination
of gemcitabine and docetaxel offers promise as a therapeutic
strategy to improve survival in advanced pancreatic cancer.
Treatment with the combination resulted in median survival and
progression-free survival comparable to that of docetaxel and
cisplatin. However, the gemcitabine-docetaxel combination was
better tolerated. Both regimens achieved longer median survival
compared to historical results with gemcitabine alone.
Gemcitabine remains one of the most widely used chemotherapeutic
agents in pancreatic cancer despite modest rates of less than
10% and median survival of less than 6 months. The combination
of gemcitabine and docetaxel has demonstrated in vitro synergy,
and docetaxel and cisplatin also have in vitro synergy.
Investigators at 17 centers compared the two docetaxel combinations
in 96 patients who had advanced pancreatic cancer. About 80%
of patients in each group had metastatic disease. Randomized
therapy consisted of gemcitabine 800 mg/m2 on days
1 and 8 plus docetaxel 85 mg on day 8 or cisplatin 75 mg/m2
day 1 plus docetaxel 75 mg/m2 day 1. Patients received
a cycle of therapy every 21 days.
The combination of gemcitabine and docetaxel proved to be
better tolerated. Patients in the gemcitabine-docetaxel group
received a median of 4 cycles of therapy, compared to 2 cycles
in the docetaxel-cisplatin group. Substantially more patients
in the docetaxel-cisplatin arm discontinued therapy because
of toxicity, and more patients in the gemcitabine-docetaxel
arm received 6 or more cycles of therapy.
Incidence of Toxicity
--
| G-D | D-C | Grade
3-4 neutropenia | 24.4% | 34.2% | Febrile
neutropenia | 6.6% | 17.1% |
Platelets | 8.9% | 2.4% | Hemoglobin | 13.4% | 14.6% | Neuropathy | 6.7% | 17.1% | Vomitin | 2.2% | 14.6% | Edema | 4.4% | 0% | Diarrhea | 4.4% | 7.3% | Discontinued-Toxicity | 15% | 26% | Treatment
6+ cycles | 44% | 29% |
G-D=Gemcitabine-docetaxel, D-C=Docetaxel-cisplatin
|
Investigators could evaluate response in 38 gemcitabine-docetaxel
patients and 31 docetaxel-cisplatin patients. Six patients
in the gemcitabine-docetaxel group achieved a partial response,
and 14 had stable disease. Five partial responses occurred
in the docetaxel-cisplatin group, and 13 patients had stable
disease.
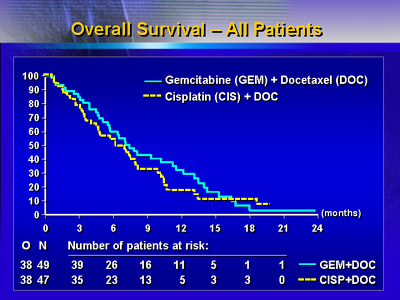
The combination of gemcitabine and docetaxel resulted in
a median survival of 7.4 months compared to 6.3 months in
the docetaxel-cisplatin group. Analysis of progression-free
survival showed a slight advantage for gemcitabine and docetaxel,
a median of 3.6 months versus 2.8 months with the docetaxel-cisplatin
regimen.
The results confirm that both combination regimens have
activity in advanced pancreatic cancer. Investigators found
toxicity to be predictable and manageable in both treatment
groups. However, the combination of gemcitabine and docetaxel
has a more favorable toxicity profile.
Gemcitabine-docetaxel combination therapy achieved a median
survival that appears to be superior to historical results
observed in patients treated with gemcitabine alone. The trial
results suggest that a randomized, controlled clinical trial
is warranted to evaluate the efficacy and tolerability of
the gemcitabine-docetaxel regimen compared to gemcitabine
monotherapy.
|