|
Several epidermal growth factor receptor
inhibitors are under regulatory review in the United States.
In phase I, II and III investigations these agents show promise
against a variety of advanced cancers as single agents and in
combination with chemotherapy. Future research will seek to
optimize combination therapy, as well as, look at these agents
in earlier stage disease.
Researchers have produced a number of antibodies that interfere
with the epidermal growth factor receptor (EGFR). They have
also produced a number of tyrosine kinase inhibitors that
act internally on the receptor. All these agents seek to stop
the signal transduction EGFR initiates after activation.
One of these agents is monoclonal antibody 225 (C225), which
Dr. Mendelsohn and colleagues described in 1983. Among other
properties, C225 binds with EGFR and inhibits activation of
receptor tyrosine kinase. This was probably the first example
of a targeted therapy against an oncogene product.
The U.S. Food and Drug Administration is currently reviewing
data on this and two other anti-EGFR tyrosine kinase inhibitors,
ZD1839 (Iressa®) and OSI-774 (TarcevaTM).
EGFR inhibition attacks many acquired capabilities of cancer
cells. This includes the ability to produce their own growth
factors, evade apoptosis, sustain angiogenesis and metastasize.
Multiple animal studies supporting the hypothesis that EGFR
inhibition can augment anti-tumor effects of conventional
chemotherapy or radiation.
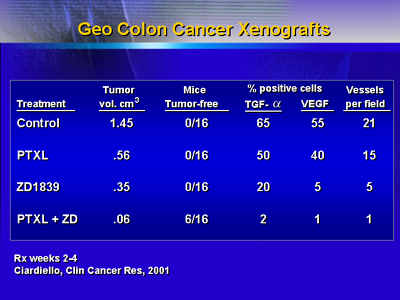
As shown in the following slide, both paclitaxel and ZD1839
reduce tumor volume somewhat in colon cancer xenografts, but
the combination produces very small tumors.
One study showed that refractory head and neck cancer patients
had partial or complete responses to C225 plus cisplatin,
including several patients who had previously failed cisplatin.
A phase II study involving 63 patients who failed to respond
to cisplatin-based chemotherapy alone. About 25% showed a
response.
A phase II trial of ZD1839 as a single agent in non-small
cell lung cancer had a 19% overall response rate. Responding
patients showed improvement in symptoms. Subsequently, investigators
conducted a 1,000-patient randomized phase III trial of ZD1839
as first-line therapy in non-small cell lung cancer. Results
will be forthcoming.
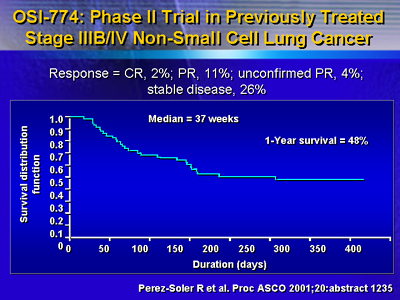
A phase II trial of OSI-774 as a single agent in previously
treated non-small cell lung cancer reported 2% complete response
and 11% partial response. The median survival was 37 weeks
and 1-year survival was 48%.
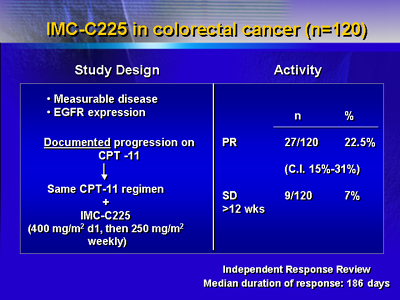
C225 has been studied in colorectal cancer. The study included
120 colorectal cancer patients who had progressed on irinotecan.
Patients received irinotecan as combination therapy with C225.
There was a 22.5% partial response rate and 7% had stable
disease.
A trial at this meeting reports
an 11% response for C225 used as a single agent in patients
who progressed on irinotecan.
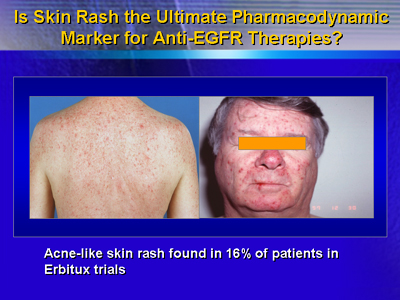
In 21 trials of C225 including 813 patients, the most common
grade 3-4 toxicities were an acne-like rash (16%) and asthenia
(9%).
A number of important challenges lie ahead. In an attempt
to optimize chemotherapy results, dozens of trials with a
C225, ZD1839 and OSI-774 are underway.
Some trials are examining the use of these agents in early
stage disease. Finally, researchers hope to eventually be
able to pre-select or predict which tumors will respond to
EGFR inhibitors. |