|
The safety and efficacy of topiramate
was investigated in a clinically based, single center, open-label
study of 135 bipolar patients. Patients received topiramate
as adjunctive treatment for 36 months. Topiramate was well-tolerated.
Clinically significant responses were observed within two to
four weeks of treatment. Patients using topiramate used less
antidepressants, benzodiazepines and mood stabilizers. The data
provided indicates topiramate is beneficial as a mood stabilizer
with antidepressant properties for the treatment of bipolar
disorder.
The novel antiepileptic drugs that include lamotrigine, gabapentin
and topiramate have expanded the pharmacotherapeutic options
for the treatment of bipolar disorder. These drugs have successfully
been used as mood stabilizers. Their improved pharmacokinetic
and tolerability profiles contribute to improved safety, efficacy
and compliance.
Dr. Hussain presented a clinically based, single center,
open-label study that was conducted to evaluate the long-term
safety and efficacy of topiramate. Topiramate was used as
adjunctive therapy for 135 patients with bipolar I (62 patients)
or bipolar II (73 patients) disorder. The duration of treatment
was 36 months. The patient population consisted of 46 males
and 89 females with a mean age of 34 years. The mean age of
onset of bipolar disorder was 21 years. The mean duration
of the current episode was 11 weeks. All participants were
previously treated with mood stabilizers and antidepressants
but failed to respond adequately. This study rated all participants
on the Hamilton Depression Rating Scale; all participants
were clinically depressed. Participants were followed for
1, 2, 3, 6, 12, 18, 24, 30 and 36 months. Topiramate was administered
at an initial dose of 25 mg/hs, rising every two days to 200
mg/hs, and later up to a maximum of 600 mg/day.
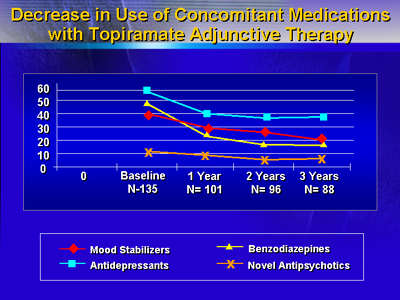
The results show that topiramate was well-tolerated. Clinically
significant responses were observed within two to four weeks
of treatment. A decrease in the use of concomitant medications
with topiramate adjunctive therapy was reported over the 36
months of treatment. Patients using topiramate used less antidepressants,
benzodiazepines and mood stabilizers. The use of novel antipsychotics
was not affected by topiramate.
Regarding the side effect of body weight, topiramate resulted
in a decrease in body weight of all participants. This weight
loss averaged 8 to 9 kg for each participant. The data provide
evidence that topiramate is an effective mood stabilizer.
Dr. Hussain noted that the reduced need for concomitant medications
suggests the possibility of decreased side effects and increased
efficacy with the use of a fewer medications. Dr. Hussain
concluded that topiramate has a beneficial use as a mood stabilizer
with antidepressant properties for the treatment of bipolar
disorder.
|