|
Investigators tried a different strategy
of starting carvedilol before discharge in hospitalized heart
failure patients. The pre-discharge carvedilol strategy significantly
increased the use of beta-blockers compared with standard
post-discharge initiation of beta-blockers. There was no excess
of side effects and no increase in length of stay. Pre-discharge
initiation of carvedilol may increase the use of potentially
life-saving therapy in this population.
Large, prospective, randomized trials show that certain
beta-blockers reduce morbidity and mortality in heart failure
patients. In addition, published guidelines recommend their
use in this setting.
However, only about 30% to 40% of eligible heart failure
patients actually receive this potentially life-saving therapy.
One problem is that a "vicious cycle" limits use
of beta-blockers in heart failure patients, Dr. Gheorghiade
said. Guidelines recommend waiting 2 to 4 weeks after hospitalization
before initiating beta-blocker therapy. However, after discharge,
the patient is often unlikely to receive a prescription. This
may be because the primary care physician is reluctant to
prescribe the medication. Some physicians may believe a prescription
is not necessary if it is not part of the discharge regimen.
New strategies to break this vicious cycle may help broaden
the use of beta-blockers in heart failure patients. One possible
way is to initiate beta-blocker therapy before discharge.
This strategy would contradict current guidelines, which suggest
waiting until after hospitalization to initiate beta-blockers.
On the other hand, data from the COPERNICUS trial suggest
carvedilol is safe and effective soon after its initiation,
even in patients who have severe heart failure.
Thus, Dr. Gheorghiade and colleagues studied the safety of
initiating carvedilol therapy prior to discharge. They initiated
a prospective, randomized, open-label trial, IMPACT-HF (Initiation
Management Predischarge: process for Assessment of Carvedilol
Therapy for Heart Failure).
The IMPACT-HF trial was a multi-center study including 363
patients who were hospitalized for worsening heart failure.
All patients had a left ventricular ejection fraction of 40%
or less. Some were randomized to pre-discharge carvedilol
started at a low dose (3.125 mg bid). The rest received standard
care, which was post-discharge beta-blocker at the physician's
discretion. The primary endpoint was the number of patients
who received beta-blocker at 60 days.
Baseline characteristics were similar between the two groups.
Baseline Characteristics
| |
Carvedilol
Predischarge
Initiation
N=185 |
Physician
Discretion
Postdischarge
Initiation
N=178 |
| Median Age |
68 (55, 77) |
66 (52, 76) |
| Male (%) |
52 |
54 |
| Caucasian (%) |
64 |
65 |
| Ischemic Etiology (%) |
44 |
48 |
| Median LVEF (%) |
25 (20, 30) |
25 (20, 30) |
(25th, 75th percentiles)
|
The most common comorbidities included hypertension, diabetes
and hyperlipidemia. Both patient groups were similar in terms
of heart failure signs and symptoms, NYHA class (mostly III-IV),
edema, and medications at baseline.
Comorbidities
| |
Carvedilol
Predischarge
Initiation
N=185 |
Physician
Discretion
Postdischarge
Initiation
N=178 |
| Hypertension (%) |
61 |
66 |
| Diabetes (%) |
37 |
41 |
Renal
insufficiency (%) |
11 |
11 |
Pulmonary
disease (%) |
15 |
12 |
| Atrial fibrillation/flutter
(%) |
20 |
24 |
Ventricular
arrhythmia (%) |
8 |
10 |
| Hyperlipidemia (%) |
29 |
30 |
|
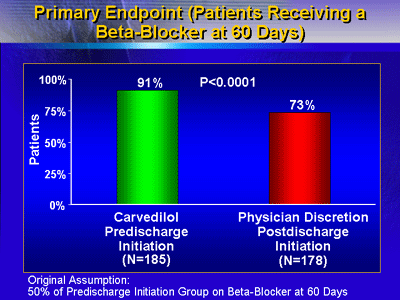
Beta-blocker Initiation
More patients in the pre-discharge carvedilol group received
a beta-blocker than in the group that received post-discharge
beta-blocker initiation at the physician's discretion. According
to Dr. Gheorghiade, 91% of patients in the pre-discharge carvedilol
group were on beta-blockers at 60 days, compared with 73%
in the post-discharge group (p < 0.0001). Mean time to
first beta-blocker initiation in the post-discharge group
was 20.3 days.
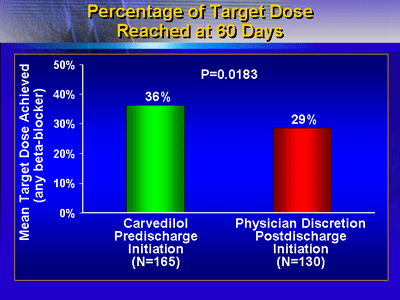
Beta-blocker Dosage
In addition, significantly more patients randomized to pre-discharge
carvedilol were receiving a higher dose at 60 days. Investigators
found that 36% of the pre-discharge carvedilol group reached
the mean target dose, compared with 29% of the post-discharge
group (p = 0.0183).
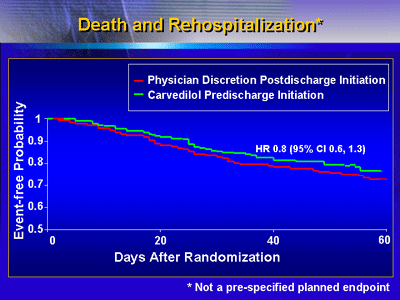
Clinical Outcomes
Pre-discharge initiation of carvedilol was not associated
with an increased risk of worsening heart failure (9%, versus
14% in the post-discharge group) or other serious adverse
events. In fact, there was a trend toward less death/rehospitalization
in the carvedilol pre-discharge group.
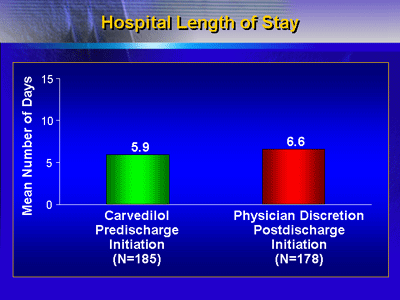
Pre-discharge carvedilol did not adversely impact length
of stay. The mean hospital stay was 5.9 versus 6.6 days for
the pre- and post-discharge groups, respectively.
These results suggest that pre-discharge initiation of carvedilol
in stabilized heart failure patients can increase use of beta-blocker
therapy. Investigators achieved this improvement without increasing
the side effect burden, or length of stay. Pre-discharge initiation
of carvedilol may be a practical approach to improving the
use of potentially life-saving therapy in this population.
|