| In isolated
diabetic rat hearts, investigators noted that the antihypertrophic
actions of B-type natriuretic peptide (BNP) are preserved. In
contrast, these mechanisms are not preserved for bradykinin,
angiotensin-converting enzyme (ACE) inhibitors, or neutral endopeptidase
(NEP) inhibitors. Administration of exogenous natriuretic peptide
may effectively prevent cardiac hypertrophy in diabetes.
Diabetes
predisposes myocardium to left ventricular hypertrophy. A
key antihypertrophic mechanism is cyclic GMP. This mechanism
mediates the endothelium-independent actions of B-type natriuretic
peptide (BNP). Cyclic GMP also appears to contribute to the
antihypertrophic effects of angiotensin-converting enzyme
(ACE) inhibitors.
Diabetes also compromises endothelium-dependent pathways.
Notably, the disease predisposes to dysfunction of endothelial
nitric oxide. This compromise can have important consequences,
since nitric oxide protects myocardium from ischemic injury.
Dr. Ritchie and colleagues have evaluated how diabetes in
the rat heart impacts the acute antihypertrophic actions of
BNP. They compared this to the actions of bradykinin, an ACE
inhibitor (ramiprilat) and a neutral endopeptidase (NEP) inhibitor
(candoxatrilat). They hypothesized that they would observe
preservation of the antihypertrophic actions of BNP, but not
of the ACE inhibitor.
Investigators isolated hearts from diabetic rats and age-matched
controls. They perfused these hearts for 90 minutes with BNP,
bradykinin, ramiprilat, candoxatrilat or vehicle. After the
first 30 minutes, they added angiotensin II, then allowed
for incorporation of a radiochemical ([3H] phenylalanine)
for 60 minutes.
They then used an assay of phenylalanine incorporation to
determine level of protein synthesis. They also used radioimmunoassays
to measure atrial natriuretic peptide (ANP) and beta-myosin
heavy chain mRNA expression.
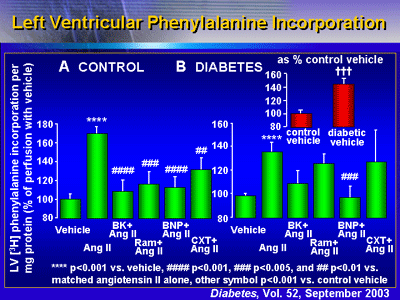
Protein Synthesis
Results show that BNP blocked protein synthesis in these
diabetic rat hearts. In non-diabetic hearts (controls), angiotensin
II increased left ventricular phenylalanine incorporation
by 170% versus vehicle alone. Bradykinin, ramiprilat, BNP
and candoxatrilat all prevented this stimulation. In diabetic
hearts, phenylalanine incorporation was 143% higher compared
with the controls. Angiotensin II further stimulated phenylalanine
incorporation 137% compared with diabetic vehicle. Importantly,
BNP abolished this hypertrophic response (p < 0.001 versus
angiotensin II alone). None of the other treatments (bradykinin,
ramiprilat, or candoxatrilat) abolished response.
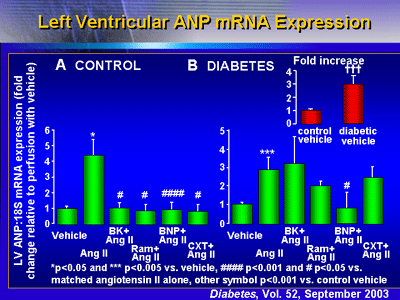
mRNA Expression
In addition, BNP also blocked ANP and beta-myosin heavy chain
mRNA expression. In control hearts, all agents prevented an
angiotensin II-stimulated increase in ANP mRNA expression.
In diabetic hearts, ANP expression at baseline was approximately
3 times higher than in control hearts. Adding angiotensin
II led to a further increase in ANP mRNA. Investigators found
BNP reduced the ANP mRNA to near baseline levels (p < 0.05;
p = 0.16 when adjusted for multiple comparisons). None of
the other agents modulated the ANP mRNA increase to any significant
degree.
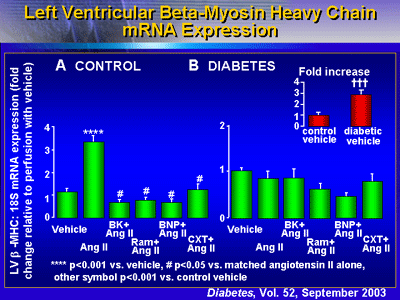
Administering BNP also appeared to reduce expression of beta-myosin
heavy chain mRNA expression, although this finding did not
reach statistical significance.
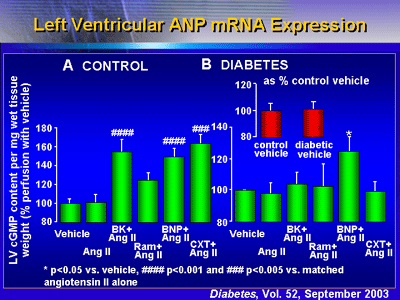
Cyclic GMP
In control hearts, all agents stimulated cyclic GMP to some
degree. However, in diabetic rat hearts, only BNP exhibited
significant stimulatory effects.
Dr. Ritchie and colleagues have shown that BNP prevents angiotensin
II-related hypertrophic responses, while bradykinin, an ACE
inhibitor and NEP inhibitor do not. In addition, BNP increased
cGMP in diabetic hearts, unlike any other treatment in this
investigation. Their findings support the hypothesis that
administering natriuretic peptide may be an effective way
to prevent cardiac hypertrophy in diabetes.
|