| Results
of the AMISTAD I and II trials suggest adenosine substantially
reduces infarct size in patients with anterior myocardial infarction.
Because of these results, adenosine merits subsequent study
in properly designed clinical trials. However, in the absence
of commercial interest, further research will likely require
public support.
The Acute Myocardial Infarction STudy of ADenosine
(AMISTAD) used myocardial perfusion imaging using Tc-99 sestamibi
to evaluate infarct size. This was the primary endpoint of
this trial, which evaluated the use of adenosine, a purine
nucleoside, as an adjunct to thrombolytic therapy.
Sestamibi imaging of infarct size often involves 2 measurements.
The measurement of the acute perfusion defect reflects myocardium
at risk. The final perfusion defect measurement, which occurs
at least 5 days later, reflects the infarct size. The difference
between these two measurements indicates the amount of myocardial
salvage.
Validation of sestamibi imaging is extensive, according to
Dr. Gibbons. There are multiple lines of evidence for validation,
including an association between smaller infarct size on sestamibi
and better patient outcomes.
Acute imaging with sestamibi can measure the area at risk
and estimate collateral flow. The final sestamibi measurement
provides time to therapy, infarct size and differential benefit
(small versus large infarct). By contrast, mortality trials
only provide time to therapy. As a result, sestamibi trials
require far fewer patients to show a benefit, and the direct
cost of sestamibi trials is much less.
Endpoints in MI Trials
| |
Sestamibi |
Mortality |
| Area at risk |
(Yes) |
No |
| Collateral flow |
(Yes) |
No |
| Time to therapy |
Yes |
Yes |
| Infarct size |
Yes |
No |
| Differential benefit |
Yes |
No |
| Patients required/arm |
140 |
10,000 |
| Cost ($ millions) |
2 |
≧ 35 |
|
Dr. Gibbons said his laboratory at Mayo Clinic has served
as the core laboratory for 14 completed sestamibi trials in
acute myocardial infarction. This included the ALIVE trial
of adenosine/angioplasty, and the AMISTAD I and II trials,
which evaluated adenosine with thrombolysis as the reperfusion
modality.
Before the randomized adenosine trials, Mayo Clinic performed
a single-center pilot study of adenosine with angioplasty.
Myocardial salvage in this study appeared to be far higher
than historical controls.
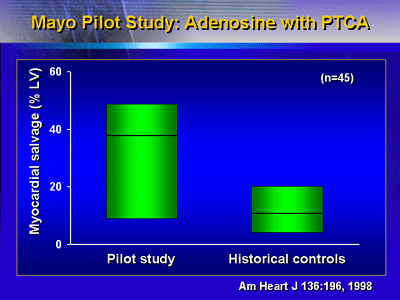
Myocardium at risk was virtually identical. Infarct size
was lower in the adenosine group, though the sponsor stopped
this pilot trial early. However, even with this small sample,
there is a strong trend favoring adenosine. These results
led to the subsequent randomized ALIVE trial.
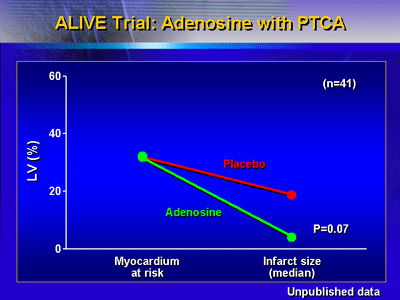
The AMISTAD trial of adenosine with thrombolysis included
236 patients with ST-elevation myocardial infarction. They
were randomized to receive intravenous adenosine 70 mcg/kg/min
or placebo beginning at the time of thrombolytic therapy and
continuing for 3 hours. The thrombolytic was tPA in 62% and
streptokinase in 38%. The primary endpoint was infarct size
by sestamibi.
In anterior myocardial infarction patients, the adenosine
group had a smaller infarct size (67% reduction, p < 0.01).
In contrast, there was no significant difference in infarct
size in the non-anterior myocardial infarction patients. Excess
bradycardia and hypotension in the inferior infarct group
represented possible adverse effects.
Dr. Gibbons said that with existing therapy, inferior infarcts
are very small. Therefore, it is far more difficult to detect
incremental benefit with adjunctive therapy.
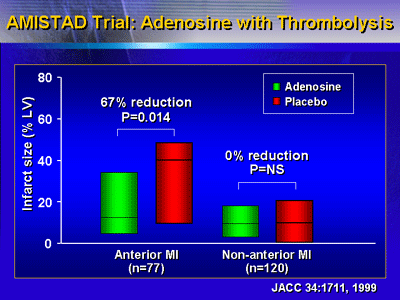
The subsequent AMISTAD II trial enrolled only patients with
anterior infarcts. This was because of the lack of benefit
and possible adverse effects in the inferior infarct group
in AMISTAD I.
The AMISTAD II trial included 2,018 patients randomized to
the same 70 mcg/kg/min dose, a lower dose, or placebo over
3 hours, started within 15 minutes of reperfusion. Investigators
followed patients for 6 months for the clinical endpoint of
combined death and new heart failure. A substudy examined
infarct size using sestamibi in 243 patients assessed after
5 days.
Unfortunately, there was no significant difference in the
clinical endpoint at 6 months. This may have resulted because
investigators decided to pool the low and high adenosine doses
for analysis, according to Dr. Gibbons. Use of streptokinase
also probably influenced the results.
Investigators found that 82% of placebo-treated patients
survived free of congestive heart failure at 6 months. In
the pooled adenosine group, survival free of heart failure
was 84%. The relative risk reduction of 11% was not significant.
When investigators re-analyzed the adenosine doses separately,
they found more of an effect in the high-dose group. However,
this analysis did not have enough power to detect a treatment
benefit.
Infarct size data were not different between placebo and
pooled adenosine doses. However, with doses re-analyzed separately,
there was a 57% reduction in the high-dose group. This was
similar to the reduction in infarct size in AMISTAD I, which
was statistically significant.
Despite some important trial design issues, the results of
AMISTAD II do seem to corroborate the substantial reduction
in infarct size investigators found in AMISTAD I. Therefore,
adenosine merits further evaluation in properly designed clinical
trials. However, because commercial interest has waned, such
a trial would probably require support from a public entity,
such as the National Heart, Lung and Blood Institute (NHLBI).
|