|
The angiotensin II receptor blocker (ARB) valsartan is as effective
as captopril in reducing mortality risk in patients at high
risk for cardiovascular events after myocardial infarction.
In addition, it is as effective as captopril in reducing risk
of subsequent myocardial infarction and heart failure in this
patient population. The combination of valsartan did not improve
survival, but increased the rate of adverse events. Investigators
believe valsartan should be an alternative to angiotensin-converting
enzyme (ACE) inhibitors in this patient population.
Many patients that survive acute myocardial
infarction have heart failure or resulting left ventricular
dysfunction. These patients have a high risk for death and
non-fatal major cardiovascular events. In clinical trials,
angiotensin-converting enzyme (ACE) inhibitors reduce this
risk by approximately 20%. The ACE inhibitors work by inhibiting
the renin-angiotensin system.
Angiotensin II receptor blockers (ARBs) also work by blocking
the renin-angiotensin system. The ARBs are newer, more specific
and may offer more complete inhibition of angiotensin II than
ACE inhibitors.
Because of this, investigators undertook a large, randomized,
multicenter international trial to find out if the ARB valsartan
provides an advantage over the ACE inhibitor captopril. Previous
investigations have proven that captopril 50 mg tid is effective
in post-myocardial infarction patients at high risk of mortality
or further morbidity.
Investigators enrolled 14,703 patients from 931 sites in
24 countries. Investigators ascertained vital status in 14,564
patients, or 99.05%.
Baseline Characteristics
| Age |
65.0 years |
| Women |
31.5 % |
| Mean BP |
123/72 mm Hg |
Killip class
I
II
III
IV |
28.0
48.3
17.3
6.4
|
| Mean LVEF |
35.4 % |
Creatinine
|
1.1 mg/dL
98 μmol/L |
| Time to randomization |
4.9 days |
| Thrombolytic therapy |
35.2 % |
| Primary PCI |
14.8 % |
| Other PCI after MI, prior to randomization |
19.8 % |
Qualifying MI site
Anterior
Inferior |
59.4 %
34.4 %
|
Qualifying MI type
Q wave
Non Q wave |
66.6
31.9
|
|
All patients had experienced a myocardial infarction within
the past 10 days. All patients had acute heart failure, left
ventricular dysfunction, or both. Randomization was to valsartan
160 mg bid, captopril 50 mg tid, or the combination of valsartan
80 mg bid plus captopril 50 mg tid.
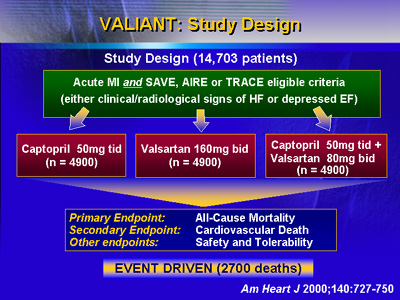
The primary endpoint of the study was all-cause mortality,
and secondary endpoints included cardiovascular death, myocardial
infarction and heart failure. Investigators also looked at
safety and tolerability.
Results showed that valsartan was as effective as the proven
dose of captopril in reducing risk of death. At the median
follow-up of 24.7 months, there were 979 deaths in the valsartan
group and 958 deaths in the captopril group (hazard ratio
1.00; 97.5% confidence interval 0.90-1.11). There were 941
deaths in the group of patients that received the valsartan/captopril
combination (hazard ratio of 0.98 compared to the captopril
group, 97.5% confidence interval 0.89-1.09). There were no
statistically significant differences in death between the
3 groups.
Investigators conducted a non-inferiority test with regard
to mortality. For valsartan versus captopril, the upper limit
of the one-sided 97.5% confidence interval was within the
prespecified margin for non-inferiority (p = 0.004). Valsartan
was also non-inferior to captopril for the combined endpoint
of fatal and nonfatal cardiovascular events (p < 0.001).
According to Dr. Pfeffer, valsartan preserves 99.6% of the
mortality benefit seen with captopril.
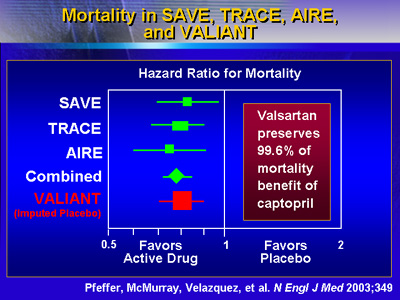
For a variety of mortality/morbidity endpoints, valsartan
was as effective as captopril, Dr. Pfeffer said. These endpoints
included cardiovascular death, cardiovascular death or myocardial
infarction, cardiovascular death or heart failure, and the
combination of cardiovascular death, myocardial infarction
and heart failure.
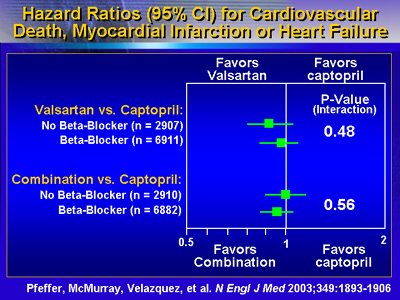
Combining valsartan with the proven dose of captopril did
not provide any further reduction in mortality, Dr. Pfeffer
reported. On the other hand, the combination of valsartan
and captopril did increase risk of adverse drug effects.
These results suggest that valsartan is a clinically effective
alternative to the ACE inhibitor captopril in post-myocardial
infarction patients at high risk of a subsequent event or
mortality. Dr. Pfeffer said clinicians now have an ARB dosing
regimen that is as effective in preserving lives and reducing
morbidity in this patient population.
Full results of the Valiant Trial are now available in the
New England Journal of Medicine (November 13, 2003, p. 1893).
|