| Carvedilol
downregulated inducible nitric oxide synthase (iNOS) and IL-6
expression in heart failure patients. This corresponded to less
augmentation of LV contractility with dobutamine following iNOS
blockade. These data suggest that an inflammatory-mediated contractile
regulatory pathway dependent on NOS may be at least partially
responsible for this beneficial effect of carvedilol.
In heart failure patients, inflammation and
norepinephrine can increase expression of myocardial inducible
nitric oxide synthase (iNOS). This can depress myocardial
contractility.
Myocardial contractility improves in patients with dilated
cardiomyopathy who receive long-term therapy with the beta-blocker
carvedilol. However, there have been no in vivo examinations
of the impact of carvedilol on expression of iNOS, and no
studies of nitric oxide-dependent beta-adrenergic inotropic
responsiveness.
Dr. Tang and colleagues hypothesized that the suppression
of myocardial iNOS expression is what leads to the recovery
of myocardial contractility that occurs after patients start
chronic beta-blocker therapy. To evaluate this, they measured
myocardial iNOS mRNA gene expression, and augmentation of
left ventricular contractility following dobutamine infusion
during iNOS blockade.
The study included stable heart failure patients with left
ventricular ejection fraction of less than 45% and NYHA functional
class II-III. There were 20 carvedilol patients in this study.
All underwent a biopsy protocol during right heart catheterization.
Twelve of these patients also underwent a hemodynamic protocol
to determine contractile response. Controls included stored
biopsy specimens from 17 beta-blocker naïve patients.
In addition, 5 beta-blocker naïve subjects underwent
both biopsy and hemodynamic protocols.
Baseline N-terminal pro-brain natriuretic peptide (NT-proBNP)
levels were slightly higher in the carvedilol group. The carvedilol
group also had higher mean left ventricular ejection fraction.
However, ejection fraction before carvedilol was similar to
the control group.
Subject Characteristics
| |
Carvedilol
(n=20) |
No
Carvedilol
(n=23) |
| Mean age (years) |
54 ± 8 |
52 ± 6 |
| Gender (% male) |
75% |
74% |
| Mean LV ejection fraction |
32 ± 8% * |
24 ± 7% |
| Etiology (% ischemic) |
40% |
52% |
| Baseline NT-proBNP (pmol/L) |
1078 ± 1205 |
714 ± 736 |
| Co-morbidities:
|
| Hypertension |
55% |
61% |
| Diabetes mellitus |
30% |
35% |
| Medications: |
| ACE inhibitors/ARBs
|
85% |
87% |
| Diuretics |
100% |
100% |
| Digoxin |
30% |
39% |
| Spironolactone |
15% |
13% |
*
Baseline mean LV ejection fraction
prior to carvedilol = 25 ± 7%
|
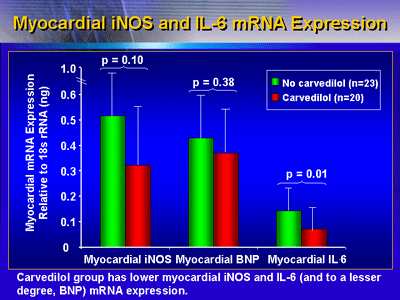
Myocardial IL-6, BNP and mRNA gene expression of iNOS were
all lower in the carvedilol group. However, the difference
was statistically significant only for IL-6.
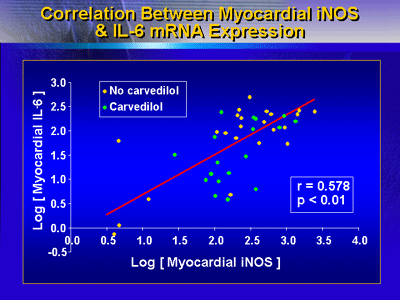
There was a direct correlation between myocardial iNOS and
IL-6 mRNA expression. This suggested that there is an association
between inflammation and myocardial iNOS expression in heart
failure.
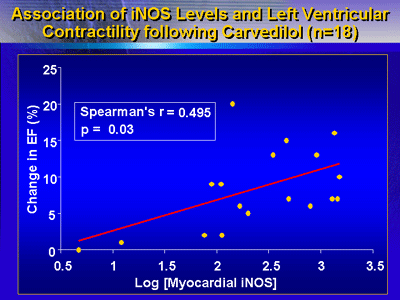
There were 18 patients in the study that had follow-up echocardiography
6 months after cardiac catheterization. In those patients,
there was an association between higher myocardial iNOS levels
and greater improvements in left ventricular contractility
after initiation of carvedilol.
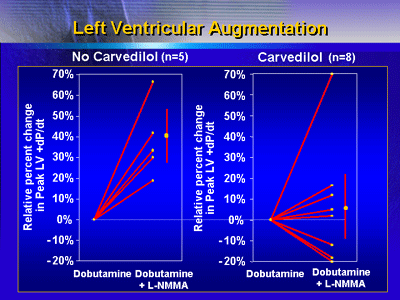
In the control patients, there was an augmentation of left
ventricular contractility of about 40%. The mean augmentation
was only 4% in the carvedilol patients. This suggests that
carvedilol therapy is associated with less functional iNOS
activity available to impair LV contractility
This study has several important limitations. Because of
its case-control design, investigators cannot demonstrate
causality. The small sample size limits the ability to confirm
that differences are statistically significant. Finally, the
determination of heart failure severity was subjective.
However, these data do suggest that carvedilol may modulate
a contractile regulatory pathway that is inflammatory-mediated
and iNOS-dependent. If so, carvedilol may act on specific
targets in the pathway, or may modulate upstream processes,
such as ischemia.
In a confirmatory study, Dr. Tang and co-investigators will
measure sequential plasma levels of nitric oxide-dependent
oxidant stress markers, such as nitrotyrosine. They will measure
these stress markers in a larger group of patients, both before
and after beta-blocker therapy.
|