| Dr. Horikawa's
work examined possible class 1c drug effects on substrates of
atrial fibrillation (AF) originating from the pulmonary vein
(PV). AF was induced in dogs with bilateral stimulation of cervical
vagal nerves after application of aconitine on a main branch
of the left or right PV. Sustained AF was induced in 21 of 27
dogs. Pilsicainide terminated AF in 20 of 21 dogs and suppressed
inducibility and sustainability of the arrhythmia, as well.
Dr. Horikawa opened by noting that researchers
have long believed that the antiarrhythmic effects of class
1c drugs on atrial fibrillation (AF) arise from prolongation
of atrial effective refractory period and reduction of conduction
velocity within the atrium; however, drug effects on the pulmonary
vein (PV) and PV-left atrial junction had not been explored
prior to the current research.
The investigators had previous experience with atrial arrhythmias
in a dog model: They had shown that an application of aconitine
to a branch of one PV can induce a regular atrial tachycardia
(called PV tachycardia) that can be converted into AF in the
majority of dogs with use of vagal nerve stimulation.
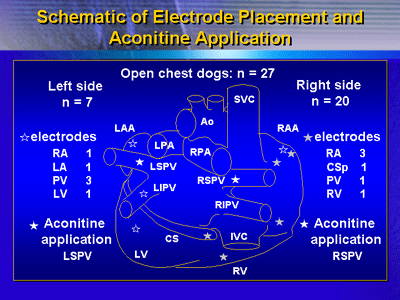
The current work used the same open chest dog model to look
for possible drug effects of pilsicainide on substrates of
AF that originate in either the left or right PV. In a total
of 27 dogs, induction of AF was done with use of bilateral
stimulation of the cervical vagal nerves after application
of aconitine on a main branch of the right or left PV (20
and 7 dogs, respectively).
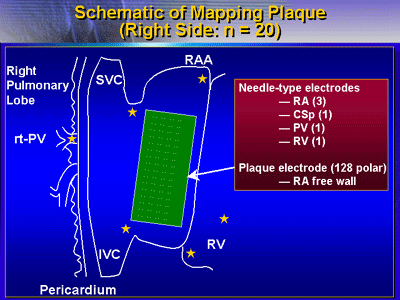
Electrophysiologic studies were conducted before and after
intravenous administration of pilsicainide at 1 mg/kg/5 min;
study setup involved electrodes in the atria and PVs, as well
as a mapping plaque with 128 electrodes placed on the surface
of the right atrium.
| |
Needle type electrodes were
inserted into the atria, PV and ventricle. |
| ・ |
A 128 polar plaque electrode was placed
on the right atrial free wall to measure the atrial conduction
velocity. |
Sustained AF originating from the aconitine-treated PV (cycle
length, 61.2 ms) was induced in 21 of 27 dogs, and PV tachycardia
that did not convert to AF was induced in the remaining 6
dogs.
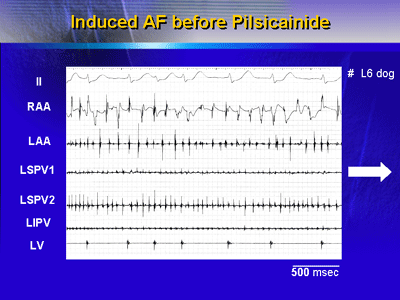
| Intracardiac
electrograms of AF before pilsicainide administration
demonstrates the short FF intervals with irregularity
in all of the recording sites. |
Pilsicainide terminated sustained AF in 20 of 21 dogs (95 percent).
The drug prolonged cycle length in the 6 cases of PV tachycardia
from 158 ms to 309 ms; PV-LA interval increased from 41.4 ms
to 57.5 ms. 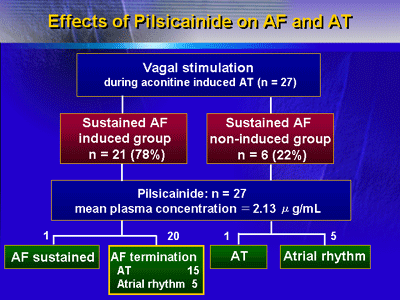
| *
|
VS converted AT into sustained
AF in 21 of 27 dogs, and in the remaining 6 dogs sustained
AF was not induced. |
| ** |
Administration of pilsicainide
terminated AF in 20 of 21 dogs. |
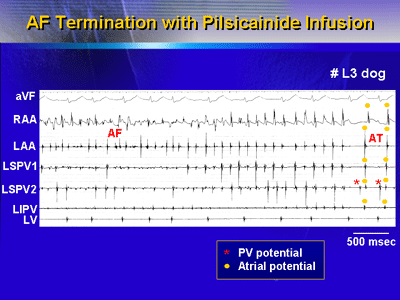
| Intracardiac
electrograms were demonstrated when AF was terminated
by pilsicainide.
Activation intervals of atria and PVs were prolonged
and the waves organized before AF was converted into
PV tachycardia. |
The findings expected for a class 1c drug were also noted:
Atrial effective refractory period increased from 90.0 ms
to 107.5 ms, and conduction velocity decreased from 73.2 cm/s
to 55.2 cm/s.
Attempts to reinduce arrhythmia with atrial burst pacing
produced sustained AF in 3 of the 20 dogs in which pilsicainide
had terminated the arrhythmia; nonsustained AF was produced
in 12 of the 20 dogs. In 6 of the 36 dogs, investigators noted
PV-left atrium (LA) dissociation before conversion to normal
rhythm.
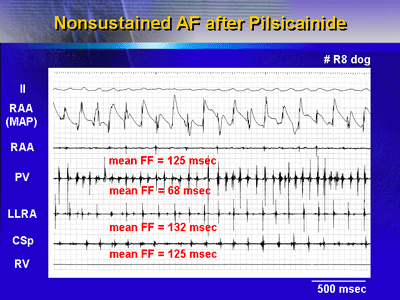
| Intracardiac
recordings during re-induced AF after pilsicainide administration
demonstrate rapid PV activation and slower atrial activation.
|
Rapid PV activation was noted in all 3 dogs in which sustained
AF was reinduced, but it was observed in only 1 of the 12
dogs in which nonsustained AF was induced. The mean plasma
concentration of pilsicainide achieved with intravenous administration
was significantly higher than the therapeutic range (2.13
μg/mL compared with 0.3 -0.9 μg/mL).
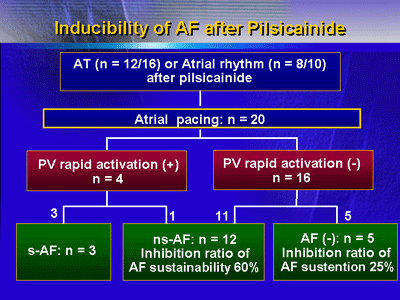
| *
|
Atrial pacing provoked
rapid PV activation only in 4 dogs, which showed AF (sustained
3, non-sustained 1). |
| ** |
Rapid PV activation
was not provoked in the remaining 16 dogs, in which sustained
AF was not induced. |
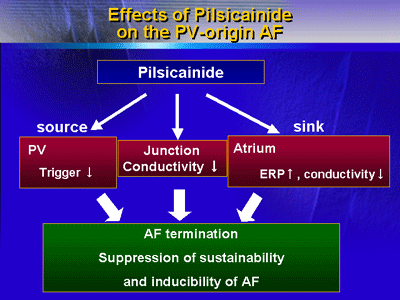
The research demonstrates in the dog model that a class 1c
drug (namely, pilsicainide) can terminate AF originating from
a PV, with antiarrhythmic effects in a variety of tissues
including the atria, PVs, and PV-LA junctions. Future research
should take these pharmacologic effects seen with AF of PV
origin into account when considering the activity of antiarrhythmic
agents.
|