| Local
controlled release of high-dose perindopril using microsphere-embedded
stents reduces inflammation and neointima formation in rabbits.
Stent-based delivery of angiotensin converting enzyme (ACE)
inhibitors may effectively suppress ACE and angiotensin II activity
in future human studies.
Research shows that angiotensin II promotes
inflammation and vascular smooth muscle proliferation. This
is especially the case after vascular injury has occurred.
These findings suggest angiotensin II may have a role in restenosis.
Furthermore, high-dose administration of angiotensin converting
enzyme (ACE) inhibitors reduces neointima formation in animal
studies.
However, recent trials have not shown that ACE inhibitors
have an effect on restenosis in humans. This may be because
investigators were concerned about side effects, and so used
systemic doses of ACE inhibitors too low to act against restenosis.
One alternative to systemic dosing of ACE inhibitors is local
delivery using drug-coated stents. With this strategy, investigators
may be able to deliver high doses locally with minimal systemic
side effects.
Accordingly, Dr. Wang and colleagues have studied stent delivery
of the ACE inhibitor perindopril in a rabbit balloon injury
model. They hypothesized that perindopril would inhibit neointima
formation and inflammation at the site of injury.
They deployed channeled stents with embedded microspheres
containing perindopril in the aortas of 8 New Zealand white
rabbits. These stents release ACE inhibitor at the rate of
2 mg per day for 28 days. Another 8 rabbits received stents
without ACE inhibitor. Subsequently, investigators subjected
all stented aortas to a balloon injury. They also fed the
animals a high cholesterol diet for the length of the study.
Investigators performed in vitro function analysis, morphological
analysis of the intima/media ratio, evaluation of local inflammatory
cell infiltrate, and evaluation of smooth muscle cell proliferation
in these rabbits.
In vitro function: Results of the analysis revealed
that the stents with perindopril delivered significant ACE
inhibitory activity. This activity slowly decreased over time
but was consistent through the final analysis at 25 days.
Degree of plaque formation: The mean intima/media
ratio was significantly smaller in the perindopril group versus
controls. This was true at both the day 7 and day 28 analyses.
Intima-to-Media Ratio
| |
Control |
Perindopril |
P value |
| Day 7 |
0.12 |
0.02 |
< 0.01 |
| Day 28 |
1.42 |
1.04 |
< 0.01 |
|
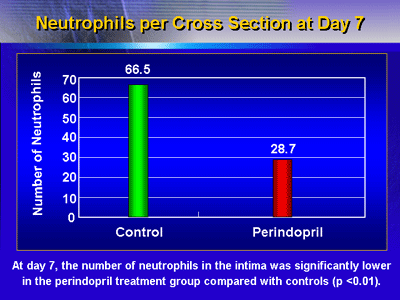
Local inflammatory cell infiltrate: At day 7, the
number of neutrophils in the intima was significantly lower
in the perindopril treatment group compared with controls
(p < 0.01).
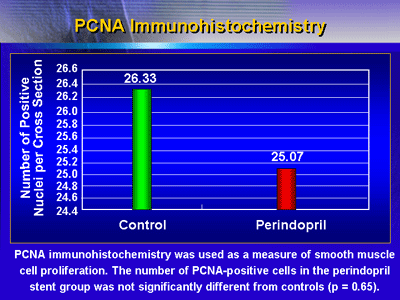
Smooth muscle cell proliferation: PCNA immunohistochemistry
was used as a measure of smooth muscle cell proliferation.
The number of PCNA-positive cells in the perindopril stent
group was not significantly different from controls (p = 0.65).
This study has shown that local and controlled delivery of
high-dose perindopril can reduce neointima formation and inflammation
without changes in smooth muscle cell proliferation. These
results support previous research suggesting that ACE inhibition
reduces neointima formation through inhibition of smooth muscle
cell migration, rather than inhibition smooth muscle cell
formation.
Dr. Wang said future human trials may evaluate the stent-based
delivery of ACE inhibitors for suppression of ACE and angiotensin
II activity.
|