| In
rat cardiomyocytes under oxidative stress, carvedilol restored
transcriptional activity of a gene that is critical for cardiac
performance. This protective effect depended on the antioxidant
action of carvedilol, not its beta blocking action. A carvedilol
metabolite without beta blocking activity also restored the
decrease in gene transcription. However, metoprolol did not
have this effect. These findings suggest that some of the beneficial
effect of carvedilol in heart failure may depend on its antioxidant
activity.
Carvedilol is a widely used beta-blocker for the treatment
of congestive heart failure (CHF). Large clinical trials have
established that carvedilol reduces mortality and improves
cardiac function for all CHF patients, particularly those
with severe disease.
The effect of carvedilol may be due not only to beta blockade,
but also to some other unknown mechanisms. One of the unique
properties of this drug is potent antioxidant activity. Oxidative
stress is an important contributor to contractile dysfunction.
Dr. Koitabashi and colleagues hypothesized that carvedilol
may exert its beneficial effect by inhibiting reduction in
transcription of a specific gene, sarco/endoplasmic reticulum
Ca2+-ATPase isoform 2 (SERCA2). Investigators previously
showed that the activity of SERCA2 correlates with cardiac
performance parameters such as systolic and diastolic function.
Furthermore, SERCA2 regulates intracellular Ca2+
handling in the failing myocardium.
Investigators wanted to know whether carvedilol attenuates
the decrease of the SERCA2 gene under oxidative stress. If
so, then the antioxidant activity of carvedilol may be an
important mechanism in preventing heart failure.
They exposed cultured neonatal rat cardiomyocytes to hydrogen
peroxide, causing oxidative stress. Then, they treated the
cells with 3 drugs: carvedilol, a metabolite of carvedilol
with no beta blocking activity, and the beta-1 selective blocker
metoprolol.
Experiments showed that hydrogen peroxide decreased SERCA2
protein mRNA levels in the rat cardiomyocytes in a dose-dependent
manner. Transient transfection assays showed that carvedilol
decreased transcription of the SERCA2 gene promoter.
Treatment with carvedilol attenuated these decreases in
SERCA2 protein levels and mRNA expression. This suggests carvedilol
could protect impairment of cardiac function that hydrogen
peroxide had induced.
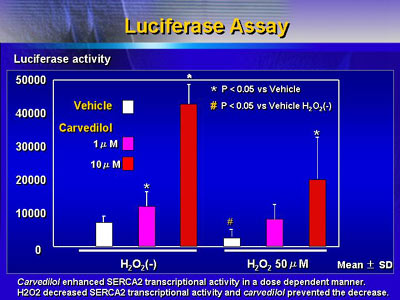
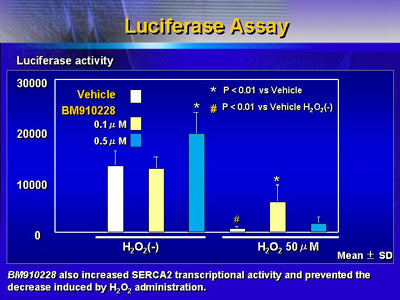
Investigators found that the non-beta blocking metabolite
of carvedilol also attenuated these hydrogen peroxide mediated
decreases.
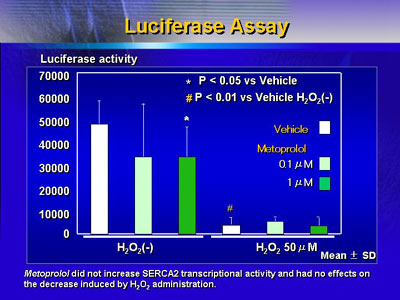
Metoprolol, a β1 selective blocker, did not exert these
effects on rat cardiomyocytes under oxidative stress. There
was no increase in SERCA2 transcriptional activity.
These findings imply that carvedilol may be a very potent
attenuator of oxidative stress from hydrogen peroxide or other
conditions that impair cardiac function, such as volume or
pressure overload.
Investigators believe that exposing rat cardiac myocytes
to hydrogen peroxide yields a very good model of heart failure.
Many conditions can induce oxidative stress within the cardiac
myocyte, including hypertension and ischemia.
Although these data are from in vitro experiments, they strongly
suggest that carvedilol has a strong antioxidant activity
in addition to its beta blocking activity. The effectiveness
of the drug in the clinical setting may be due at least in
part to its antioxidant activity.
|