|
Dr. Cohen presented findings from the
Treatment of Enoxaparin and Tirofiban in Acute Myocardial
Infarction (TETAMI) trial, which was designed to evaluate
therapy with a low-molecular-weight form of heparin (enoxaparin)
and a platelet IIb/III receptor blocker (tirofiban) for patients
who had not been given reperfusion therapy. Data did not reveal
any statistically significant differences between patients
treated with enoxaparin vs. unfractionated heparin or with
tirofiban vs. placebo. However, subgroup analysis indicated
that enoxaparin might have benefit for patients with Killip
class I infarcts (the smallest infarctions). Both enoxaparin
and tirofiban might have benefit for patients within 12 hours
of symptom onset.
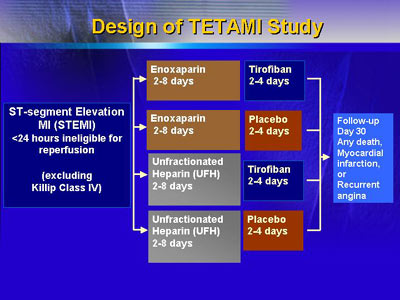
The primary goal of the Treatment of Enoxaparin and Tirofiban
in Acute Myocardial Infarction (TETAMI) trial was to see whether
enoxaparin, a low-molecular-weight form of heparin, might
have an advantage over unfractionated heparin for patients
who had not been given reperfusion therapy because of duration
of symptoms or inability to start reperfusion within 12 hours
of symptoms onset. The secondary goal was to evaluate the
efficacy of tirofiban, a platelet IIb/III receptor blocker,
vs. placebo for the same patient group.
Thus, the study design required four distinct treatment
arms. Overall, the study enrolled 1,224 patients. The four
arms were equivalent in key traits, including comorbid conditions.
With an average age of roughly 63 years, study patients were
somewhat older than a typical group given reperfusion therapy.
The presence of Q waves at enrollment for more than 66% of
patients indicated that they were, indeed, past the earliest
period of their infarction. The average time-to-treatment
was 16-17 hours after symptom onset (cut-off for entry was
24 hours). The major reasons given for lack of reperfusion
therapy was usually late arrival (greater than 12 hours after
symptom onset), although some patients had initially presented
at less than 12 hours but were referred because of unavailability
of reperfusion at original facility.
Data analysis showed that the incidence of the primary clinical
endpoint was 17.3% for unfractionated heparin and 15.4% for
enoxaparin. The difference was negligible for tirofiban, with
an incidence of adverse endpoint for 16.6% for tirofiban vs.
16.4% for placebo-treated patients.
|
TETAMI: Results at 30 days
| Endpoints
|
Enoxaparin |
Enoxaparin
+
Tirofiban |
UFH |
UFH
+
Tirofiban |
| Randomized
patients |
|
Death/re-MI/
Recurrent angina |
|
Death/re-MI |
|
|
|
|
|
| Treated
patients |
| |
Major hemorrhage |
| |
Hemorrhagic stroke |
|
|
|
|
|
|
Dr. Cohen identified two points for discussion from the
statistically similar results on both agents. First, he noted
that investigators did some post-hoc analyses to see whether
any subgroups of patients within the larger, heterogeneous
study population might have benefited from one or both agents.
Second, he thought it merited discussion to explore the limitations
of the TETAMI study in light of design of future trials for
this difficult and diverse group of patients.
The researchers conducted two subanalyses regarding enoxaparin.
They found evidence that there might be an outcome advantage
with enoxaparin for patients with Killip class I infarcts
(those with the smallest, lowest risk infarcts). Adverse rate
was 13.5% for enoxaparin compared with 16.2% for heparin.
In addition, there was a suggestion that there might be a
benefit with enoxaparin for patients who presented earlier
in the clinical course (within 12 hours of symptom onset).
Subanalysis regarding use of tirofiban indicated there might
be an advantage with tirofiban for patients who presented
within 12 hours of symptom onset.
Beyond an effort at defining and evaluating results for
patient subgroups is an effort to appreciate the limitations
of any study dealing with such a heterogeneous, high risk
patient population. Dr. Cohen thought that interpretation
of the TETAMI design and findings may point the way to design
of future trials for patients for whom reperfusion is not
an option. Although the TETAMI trial was the first major study
for nonreperfused patients, it will not be the last.
|