|
Dr. Granger presented the 2 trials of
the Complement and Reduction of Infarct Size After Angioplasty
or Lytics (CARDINAL) program, which evaluated whether a complement
inhibitor (pexelizumab) could decrease infarct size or improve
clinical outcome when used as an adjunct with reperfusion therapy.
Pexelizumab had no effect on outcomes with thrombolysis as primary
therapy (COMPLY trial); however, mortality in patients who received
angioplasty (COMMA trial) was significantly reduced with bolus/infusion
use of pexelizumab.
Dr. Granger set up discussion of the 2 trials of the Complement
and Reduction of Infarct Size After Angioplasty or Lytics
(CARDINAL) program by noting that mortality and development
of heart failure remain common after myocardial infarction,
even after optimal reperfusion was achieved. No trial in the
last 10 years has shown a reduction in mortality with an adjunct
treatment for reperfusion, according to Granger, and researchers
have remained focused on possible ways in which the cellular
damage and inflammation associated with reperfusion techniques
can be reduced or eliminated.
The CARDINAL program is a phase II study designed to test
whether adjunct use of a complement inhibitor (pexelizumab)
could reduce infarct size or improve clinical outcome after
reperfusion. The program consisted of 2 trials: the COMPLY
trial, in which reperfusion was achieved with thrombolysis,
and the COMMA trial, in which reperfusion was achieved with
angioplasty.
Characteristics of CARDINAL patients and their clinical
presentation are shown in the table below.
Characteristics of patients
in the COMPLY
and COMMA trials(CARDINAL Program)
|
Note that
clinical presentations are the same for both
trials.
|
| |
COMPLY
(thrombolysis) |
COMMA
(angioplasty) |
| Patients (n) |
920
|
814 |
| Median age (years) |
60 |
61 |
| % of patients female |
30 |
25 |
・Clinical characteristics
of acute myocardial infarction
・Within 6 hours of first symptoms
・At least 2 mm ST-segment elevation in 2 contiguous
leads or
new onset left bundle branch block
|
Because pexelizumab's point of action in the complement cascade
is at generation of C5a and C5b-9 fragments (which have strong
inflammatory activity), there is hypothetical preservation
of anti-bacterial activity through C3b, which is produced
earlier in the cascade. However, both trials excluded patients
with deficiencies of white blood cells or evidence of infection.
Each trial had 3 treatment arms from which data were generated:
placebo, pexelizumab 2.0 mg/kg bolus over 10 minutes, and
pexelizumab bolus followed by 1.0 mg/kg infusion over 20 hours.
The primary endpoint was infarct size determined by the
area under the curve for CK-MB level with use of a log rank
test. Secondary endpoints were clinical composite at 90 days
(death, development of heart failure, cardiogenic shock, or
stroke), as well as incidence of each clinical component.
Data from the trial with thrombolysis as primary therapy
(COMPLY trial) show no difference in infarct size or clinical
composite with use of bolus or bolus/infusion use of pexelizumab.
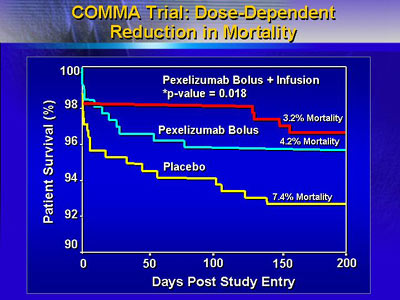
Data from the trial with angioplasty as primary therapy
(COMMA trial) show that there was no drug effect on infarct
size or clinical composite endpoint at 90 days. However, the
investigators were pleasantly surprised to see a statistical
difference in all-cause 90-day mortality (1.8% for bolus/infusion,
4.1% for bolus, and 5.9% for placebo).
Data from both trials showed that pexelizumab is effective
as a complement inhibitor (complete inhibition of hemolysis
for 4 hours with bolus, 24 hours with bolus followed by infusion).
Safety data indicate it is well tolerated at the dosages used
in the trial.
Dr. Granger said that the findings of safety and efficacy
in reducing mortality (both trials and COMMA trial respectively)
suggest that complement inhibition may have a clinical benefit
through a mechanism other than reduction in volume of infracted
myocardium. Additional research is indicated.
|