|
Dr. Steinhubl presented 1-year results
of this study. It is the first randomized study to evaluate
long-term use of clopidogrel in patients who undergo percutaneous
coronary intervention. Extending clopidogrel therapy for 1 year
resulted in a 27% relative reduction in risk of death, heart
attack or stroke.
Despite continuing improvements in treatment and prevention,
atherosclerotic coronary artery disease (CAD) remains the
single most important cause of mortality in all industrialized
nations.
Percutaneous coronary intervention (PCI) is one of the most
frequently used treatments for this condition. The procedure
is performed in approximately 5 million patients worldwide
every year. Clopidogrel, along with aspirin, is part of routine
treatment for all patients who undergo PCI today, but it typically
limited to short-term therapy of only 2 to 4 weeks.
Until recently, physicians have not fully appreciated the
long-term high risk of atherosclerotic events in patients
who receive percutaneous intervention. The first randomized
trial to evaluate the effect of long-term clopidogrel treatment
in a PCI population is Clopidogrel for the Reduction of Events
During Observation (CREDO).
99 hospitals in the United States and Canada participated
in this double blind, randomized trial. Investigators evaluated
extended clopidogrel therapy in the prevention of long-term
thrombotic events of a PCI population. They randomized 2,116
patients from the United States and Canada to a treatment
arm or placebo.
The treatment arm included a 300 mg loading dose of clopidogrel
3 to 24 hours before the procedure, while the control arm
received placebo. Both groups received clopidogrel and aspirin
at the time of the procedure and for 28 days after that. Then,
the treatment arm continued on clopidogrel and aspirin for
a total of 1 year of treatment, while the control group received
placebo and aspirin for the same time period.
The primary outcome measure was the composite rate of death,
myocardial infarction or stroke. In this patient population,
investigators reported a 27% relative reduction in risk of
death, myocardial infarction or stroke among the patients
who received long-term clopidogrel therapy (p=0.02).
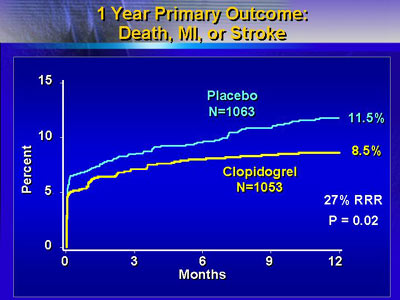
This represented a 3% absolute reduction in risk, from 11.5%
to 8.5%. Importantly, the benefit occurred in all subgroups
studied. This included women vs. men, diabetics and non-diabetics,
patients with acute coronary syndromes and those without,
and patients who received glycoprotein IIb/IIIa inhibitors
at the time of the procedure, and those who did not.
In addition, the degree of benefit in the combined endpoint
was nearly identical to the benefit seen in the individual
endpoint components. There was a relative risk reduction of
approximately 25% for rate of death, myocardial infarction
and stroke.
There was no difference between groups in incidence of minor
bleeding. However, there was a non-significant trend toward
an increase in major bleeding. Almost all of the major bleeding
occurred in patients who had an invasive procedure. About
4% of patients in both clopidogrel and placebo groups received
a coronary artery bypass graft. Over half of all patients
undergoing bypass had a diagnosis of major bleeding.
Dr. Steinhubl said the clinical implications of these results
are potentially enormous. If physicians applied these results
to the millions of patients who undergo percutaneous intervention,
clopidogrel could save patients from 50,000 heart attacks,
strokes or deaths each year. In addition, even longer treatment
with clopidogrel could lead to even greater benefit.
|