|
3 years of pravastatin treatment reduced
risk of coronary disease in the elderly. This finding suggests
that clinicians should not hesitate to extend statin treatment
beyond middle-aged persons to older individuals.
Currently, there are approximately 150 million individuals
worldwide aged 65 or older. This group is expected to swell
to 300 million within 30 years. Accordingly, physicians can
expect to see a substantial increase in the number of heart
attacks, strokes, disability, dependence on society, and dementia.
One of the most pressing questions in statin trials is the
effect on the elderly. Statins clearly reduce morbidity and
mortality in middle-aged individuals. However, their efficacy
and safety is not fully established in older patients.
The PROSPER study (PROspective Study of Pravastatin in the
Elderly at Risk) was an international trial including one
center each in Scotland, Ireland and the Netherlands. Investigators
designed this study to determine whether they could reduce
vascular risk in the elderly using statin therapy.
Researchers recruited 5,804 individuals, including 3,000
women and 2,804 men, between the ages of 70 and 82 years.
They randomized the patients to receive either pravastatin
40 mg/day or placebo.
Approximately one-half of the patients had existing vascular
disease, and the other half were at very high risk of vascular
disease because of risk factors including diabetes, hypertension
or smoking. All individuals had good cognitive function at
beginning of trial.
Because of the advanced age of the trial subjects, investigators
agreed to limit the trial length to no more than 3.5 years
of follow-up. By comparison, most large, randomized statin
trials follow patients for 5 years or longer. Therefore, PROSPER
investigators did not expect to see a mortality benefit. Instead,
they chose to evaluate the ability of statins to reduce morbidity.
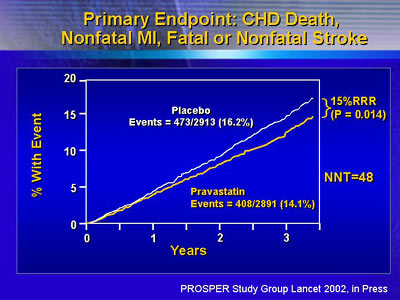
The primary endpoint of the trial combined incidence of coronary
death, myocardial infarction and stroke (fatal or non-fatal).
In addition to lowering LDL cholesterol by 34%, pravastatin
lowered the composite primary endpoint of vascular events
by 15%. There were 408 events in the pravastatin group, compared
with 437 in the placebo group (p = 0.014). Dr. Shepherd said
they would need to treat 48 individuals to prevent one coronary
death, myocardial infarction or stroke.
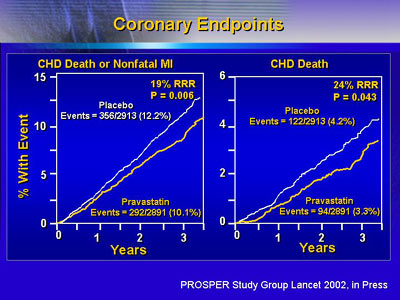
The greatest benefit was seen in coronary vessels. There
was a 19% reduction in fatal and non-fatal myocardial infarction,
and a 24% reduction in deaths from coronary heart disease
alone.
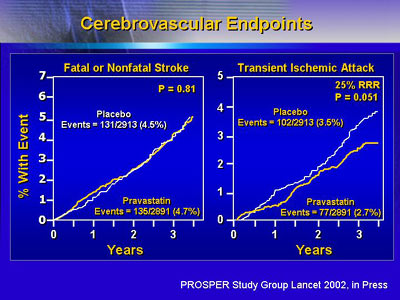
Previous statin trials showed it is possible to prevent
stroke with statin treatment. However, this benefit did not
appear until after 5 or more years of treatment. The PROSPER
trial, which included a mean of 3.2 years of patient follow-up,
showed no effect on stroke risk. This suggests that it is
not possible to prevent stroke in a short treatment period
in individuals who are at higher risk of such events.
Because there was no benefit in reduction of stroke, investigators
felt it would be unlikely to see an improvement in cognitive
function. In fact, treatment with pravastatin did not attenuate
or accelerate the progressive decline of cognitive function
that occurs in old age.
Safety analysis showed that adding pravastatin to other
drugs did not increase the risk of side effects. Patients,
on average, were taking 3.6 different drugs per day, and as
many as 16 per day. Despite that, there was no increase in
risk of myopathy, no reports of rhabdomyalysis, and no increase
in liver function abnormalities.
Consequently, Dr. Shepherd and colleagues believe PROSPER
is good news for the elderly. The study results show that
statin treatment as currently applied to middle aged individuals
is equally applicable to older persons.
|