| Investigators
have conducted the first randomized, controlled study of genetic manipulation
of coronary artery bypass grafts. They found a genetic decoy that
interferes with an important transcription factor and might reduce
morbidity and mortality in vein graft failure.
Neointimal hyperplasia, and the subsequent acceleration of atherosclerosis,
result in a high rate of vein graft failure (30% to 40%). One potential
approach to counteract this process is gene suppression using the
E2F decoy. This is an oligonucleotide that binds and inactivates
the E2F cell-cycle transcription factor.
Blocking this transcription factor prevents proliferation and growth
of vascular cells and subsequent atherosclerotic lesions. In addition,
the genetic intervention stimulates wall thickening of the graft.
Thus, the vein works more like an artery and maintains better patency
over time.
A 1999 Lancet article describes the pre-clinical experience with
E2F decoy. Researchers reported that this agent inhibited vascular
smooth muscle proliferation, and prevented vein graft disease in
leg bypass graft procedures.
In a follow-up study, German investigators randomized 200 patients
undergoing coronary artery bypass graft procedure to receive E2F
decoy or placebo. At this meeting, those researchers reported a
significant 30% relative reduction in the composite of vein graft
failure and death for the group that received E2F decoy.

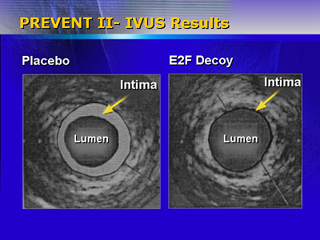
Intravenous ultrasound revealed that a 30% decrease in vessel wall
volume in the E2F decoy group. Investigators said this supports
the hypothesis that this treatment modifies the biology of the vein
graft, making it more resistant to atherosclerosis.
This treatment was safe and well tolerated, and there was no difference
in adverse events between groups. The graft is treated with the
E2F decoy ex vivo, so the patient has minimal systemic exposure.
There was a trend suggesting fewer major adverse cardiac events
in the E2F decoy treatment group, but the study did not have the
statistical power to confirm this difference. Next, investigators
plan to conduct a pivotal phase III study in coronary artery bypass
graft patients.
|