The sirolimus-eluting
stent abolishes in-stent restenosis in patients with long lesions
in small vessels. These patients are at very high risk of restenosis.
Event-free survival was 96% at 9 months. These results suggest
the positive benefits of the sirolimus-eluting stent extend
to patients with long lesions in smaller vessels.
The main limitation of coronary revascularization is the
need for repeated treatment because of restenosis of the vessel.
One potential approach to avoiding retreatment is to use a
stents that elute sirolimus, which inhibits lymphocyte and
smooth muscle cell proliferation.
Recent trial results show that the sirolimus-eluting stent
is promising for preventing neointimal events and avoiding
major cardiac events. Investigators reported results of the
Sirolimus-Eluting Stent (SIRIUS)
trial in September 2002. The randomized study included 1,058
patients who received either a sirolimus-eluting stent (Cypher)
or a bare metal stent (Bx Velocity). At 8 months, angiographic
follow-up revealed a 3.2% rate of in-stent restenosis, a 91%
reduction versus the control arm. In-lesion restenosis was
8.9%, or 75% lower than in the control arm. Event-free survival
at 9 months was 92.7%, versus 80.7% for the bare metal stent
group (p<0.001).
The Canadian Multi-Center, Randomized, Double-Blind
Study of the Sirolimus-Eluting Stent
(C-SIRIUS) included 100 patients with long stenoses in small
vessels. Each patient had a single de novo coronary
lesion between 15 and 32 mm in length. The vessel diameter
was between 2.5 and 3.0 mm. All patients had angina or documented
silent ischemia.
Investigators randomized these 100 patients to receive either
the sirolimus-eluting stent or an uncoated Bx Velocity stent.
All patients received clopidogrel daily for 2 months. Angiographic
follow-up was at 8 months and clinical follow-up at 9 months.
The primary endpoint was in-lesion minimal lumen diameter
at 8 months.
Baseline demographics were comparable between the two groups.
Approximately 69% were men and the mean age was about 60 years.
Slightly fewer patients in the control group received multiple
stents (34% versus 46%) but the difference was not statistically
significant.
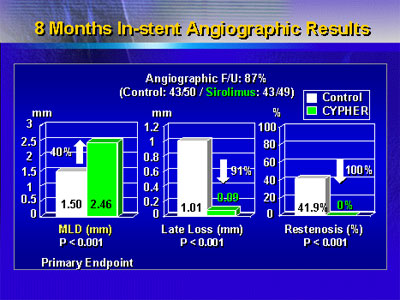
Dr. Schampaert reported that at 8 months, the sirolimus
stent treatment resulted in a 40% increase in minimal lumen
diameter. This result was statistically significant. In addition,
there was a statistically significant reduction in late loss,
and not a single case of restenosis in the sirolimus stent
group.
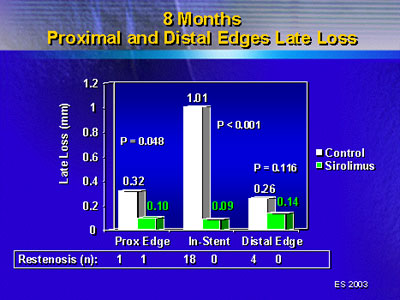
In-lesion angiographic results showed a similar trend. This
area includes the stent plus a region 5 mm proximal and distal
to the stents. There was a 35% increase in minimal lumen diameter,
87% late loss reduction and 95% restenosis reduction. That
single case of restenosis in the sirolimus-eluting stent group
occurred at the proximal edge of the lesion. It occurred even
though there was a significant reduction in late loss for
that segment.
There were few major adverse cardiac events in either group.
Investigators reported these events occurred in 2 patients
(4%) in the sirolimus-eluting stent group and 9 patients (18%)
in the control group (p=0.05). There were no deaths or Q-wave
myocardial infarctions in either group.
|
MACE at 9 Months (Clinical follow-up: 100%)
| |
Control Stent
(n=50) |
Sirolimus-Eluting
Stent (n=50) |
p value |
| MACE * n
(%) |
9
(18%) |
2
(4%) |
0.05 |
| Death |
0 |
0 |
1.00 |
| Q MI |
0 |
0 |
1.00 |
| Non-Q MI |
2 |
1 |
NS |
| TLR-PCI |
9 |
2 |
0.05 |
| TLR-CABG |
0 |
1 |
NS |
| TVF ** |
9 |
2 |
0.05 |
| Subacute Closure |
0 |
1 |
NS |
| Late Stent Thrombosis |
1 |
0 |
NS |
* MACE: Death, MI (Q or WHO Non-Q),
emergent CABG, clinically driven TLR
** TVF: Death, MI, or TVR at 9 M F/U
|
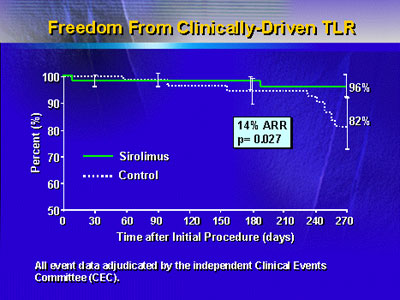
Survival free of a clinically driven target vessel revascularization
was 96% in the sirolumis-eluting stent group, versus 82% in
the bare metal stent group. This represented an absolute risk
reduction of 14% (p=0.027).
The small sample size of 100 patients limits this study.
However, Dr. Schampaert said the sirolimus-eluting stent does
appear to prevent in-stent restenosis in this very high risk
group. These results now extend the positive benefits that
occurred in earlier trials to this patient population.
The sponsor of the C-SIRIUS study was Cordis, Johnson &
Johnson Company, Canada.
|