Investigators
described a phase II double-blind, randomized trial of therapy
with endothelial growth factor for 105 patients with disabling
unilateral intermittent claudication. Patients received injections
directly into the skeletal muscle of the affected leg. Clinical
endpoints such as peak walking time and exercise treadmill testing
were evaluated at baseline, 12 weeks, and 26 weeks. There were
no differences in any of the outcomes among the 3 treatment
arms (placebo, low-dose, and high-dose gene therapy). Gene therapy
was found to be safe, with no major safety concerns identified
through a full 52 weeks of follow-up.
Dr. Rajagopalan opened by noting that the level of disability
associated with severe intermittent claudication is comparable
with that of Class III congestive heart failure. About two
thirds of patients have difficulty walking the equivalent
of half a city block.
The goal of the Regional Angiogenesis with Vascular Endothelial
Growth Factor in Peripheral Arterial Disease (RAVE) trial
was to evaluate whether intramuscular delivery of endothelial
growth factor would trigger angiogenesis in the affected limb
and improve peak walking time, Gardner protocol exercise treadmill
testing, or both in patients with severe disease.
Vascular Endothelial Growth Factor 121 was delivered intramuscularly
as a transgene on a replication-deficient adenovirus, a technique
that had achieved angiogenesis in animal models of coronary
and peripheral ischemia.
Inclusion criteria were designed to identify people with
severe, primarily unilateral disease with preserved inflow
into the affected leg. Eligible patients had two baseline
exercise treadmill tests with a peak walking time of 1 to
10 minutes and with the two results within 20% of each other.
Potential subjects were also required to have adenoviral titers
(Immunoglobulin M) of less than 1:50.
Exclusion criteria included general contraindications to
growth factor treatment such as history of cancer or diabetic
retinopathy; patients who were using either cilostazol or
pentoxyfylline were only eligible if they discontinued the
medication 1 or more weeks prior to baseline screening.
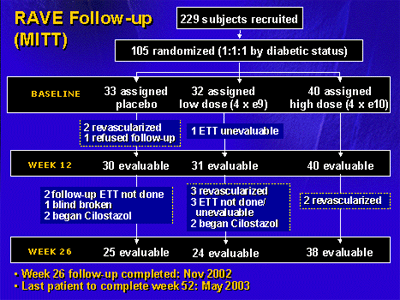
As shown in the flowchart, 229 people were recruited for
the study, and 105 were randomized to placebo, low-dose gene
therapy, or high-dose gene therapy based on diabetic status.
Enrollment demographics showed that most patients had multiple
risk factors for vascular disease, including diabetes (27%,
25%, and 30% for the 3 arms, respectively), active smoking,
hypertension, and hypercholesterolemia.
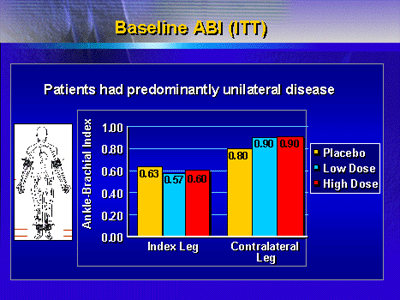
Baseline circulation testing with use of an ankle brachial
index showed that all three groups did, in fact, have primarily
unilateral disease. Average index values for the affected
leg were roughly 0.60, whereas values for the other leg were
significantly higher (0.80 for placebo group and 0.90 for
low-dose and high-dose gene therapy groups).
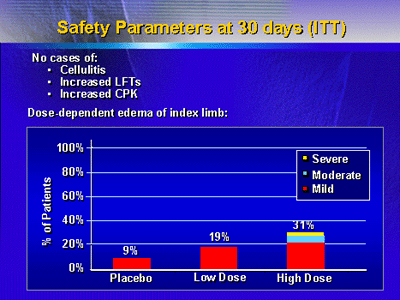
Safety data at 30 days after treatment were excellent, with
no cases of cellulitis in the injected leg or increased liver
function tests or increased skeletal muscle enzymes on blood
testing. A dose-dependent edema in the affected leg was noted.
Both placebo and low-dose groups had only mild edema, whereas
there were some cases of moderate or severe edema in the high-dose
group. There were no cases of bilateral edema.
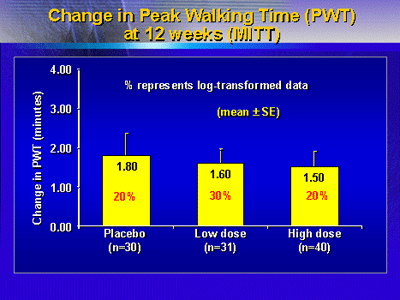
Assessment of clinical endpoints at 12 weeks produced disappointing
results: All three groups showed comparable increases in peak
walking time and claudication onset time. Ankle brachial index
values at rest and after exercise showed no change over time
for any of the 3 treatment arms. A clinical measure of level
of function showed that all 3 groups improved substantially
from baseline. The same patterns were observed at 26 weeks.
The RAVE trial is the largest placebo-controlled adenoviral
gene therapy trial to date for patients with cardiovascular
disease. Gene therapy was shown to be safe through 52 weeks
of follow-up, with the notation that edema was more common
and somewhat more severe in the high-dose group. Perhaps most
unexpected was the finding of a large, positive placebo response.
There were no indications of treatment efficacy over placebo
in any of the primary or secondary endpoints from baseline
through 26 weeks.
|