Investigators
described a double-blind, randomized trial of therapy with endothelial
growth factor for 80 patients with stable angina who had exhausted
other treatment options. Patients received injections directly
into myocardium with reversible ischemia. Imaging studies were
done at baseline and 3 months to evaluate perfusion, and evaluations
were blinded both to treatment and time point. Gene therapy
was associated with improved perfusion and decreases in number
of anginal episodes per day and use of nitroglycerine. No gene-related
adverse side effects were seen.
Although several small studies have shown promising results
for gene therapy with endothelial growth factors, the safety
and benefit of such treatment has remained unproved. The goal
of the Euroinject One Trial was to evaluate such therapy (with
plasmid-delivered VEGF-A165) in a double-blind,
randomized controlled trial of patients with stable angina
who had exhausted all other treatment options.
Investigators at multiple centers enrolled a total of 80
patients: Half received 10 injections of growth factor into
areas of documented left ventricular reversible ischemia,
whereas the other half received the same plasmid but with
the sequence for VEGF-165 deleted (see graphic of plasmid
structure).
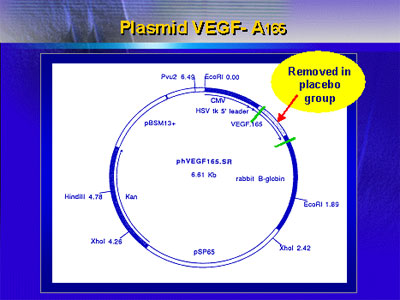
Characteristics of the two treatment arms were similar,
with average age roughly 60 years, ejection fraction roughly
40-50%, and no differences in prevalence of diabetes, prior
myocardial infarction, or prior revascularization procedure.
The primary endpoint was ventricular perfusion assessed
with Spect imaging; the three experts who read all scans were
blinded to treatment arm and time point (latter, baseline
versus follow-up). Secondary endpoints were clinical outcomes
such as number of anginal episodes per day and use of nitroglycerine.
When perfusion results were analyzed on an intention-to-treat
basis, there was a large difference in improvement between
actively treated patients and placebo treated patients. However,
a relatively large placebo effect was noted. When patients
who had coronary events during the follow-up period were excluded,
the difference favoring gene therapy was enhanced.
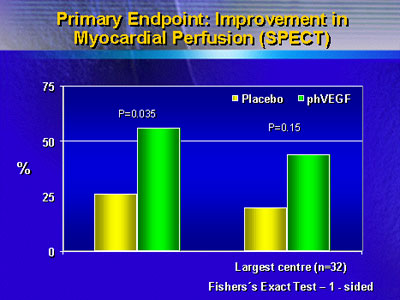
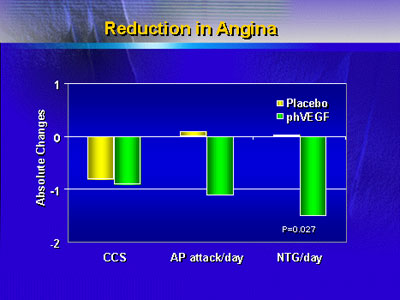
Assessment of clinical outcomes also showed benefits of
gene therapy: There was a significant difference in daily
usage of nitroglycerine and a trend toward fewer anginal attacks
per day, both favoring active treatment.
Plasma growth factor and C-reactive protein levels rose
in both groups at 1 week after injection, probably due to
injection-related trauma, but normalized afterward in placebo-treated
patients. Growth factor levels remained high at 2 and 4 weeks
in the active treatment group, confirming that transfection
occurred in these patients. C-reactive protein levels fell
in the active treatment group, indicating that transfection
did not cause an inflammatory process in the treated myocardium.
In an additional analysis, investigators found that levels
of stem cells involved in growth of new blood vessels (CD34
cells) were increased after injection in the active treatment
group but not in patients who received placebo injections.
The investigators concluded that intramyocardial injection
with growth factor (specifically, VEGF-A165) improved
perfusion of treated areas as well as clinical outcomes such
as nitroglycerine use; there was no evidence of adverse side
effects. In combination with the findings of other studies,
gene therapy merits further investigation to evaluate whether
it may become a routine treatment in the future for patients
with severe coronary artery disease.
|