Investigators
reported results from an open-label, randomized controlled trial
of continuously infused glucose, insulin, and potassium in patients
with acute myocardial infarction admitted to a single center
for primary angioplasty. Infusion resulted in a non-significant
reduction in 30-day mortality for patients as a whole, but there
was a significant reduction among patients in Killip Class 1.
Infusion was also associated with improvements in infarct size
based on serial enzyme levels and left ventricular function.
Although research has been conducted since the 1960s with
combination infusion of glucose, insulin, and potassium in
the setting of acute myocardial infarction, the question of
effect on 30-day mortality remains unresolved. The Glucose-Insulin-Potassium
Study (GIPS) was designed to address this question with a
large, open-label, randomized, controlled clinical trial involving
patients admitted with acute myocardial infarction to a single
center for primary angioplasty.
The 940 patients enrolled in the study all had acute ST-segment
elevation infarctions with symptom onset of 24 hours or less
before admission. Major exclusion criteria were pretreatment
with a thrombolytic agent or presence of an illness associated
with lowered life expectancy. Baseline characteristics were
similar for the infusion and control patients with the exception
of a smaller proportion of men in the infusion group.
A specific regimen of high-dose, continuously infused glucose,
insulin, and potassium was used for patients in the active
treatment arm. The primary endpoint was 30-day mortality.
The three secondary endpoints were a composite of 30-day mortality,
recurrent infarction, or repeat revascularization procedure,
high enzymatic infarct size, and left ventricular ejection
fraction less than 30 percent.
Post-angioplasty results were similar for the two groups,
with no significant differences observed. When the 30-day
mortality data were analyzed, there was no significant difference
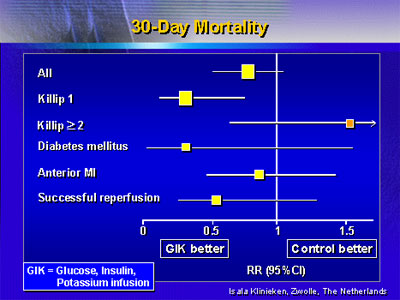
between treatment arms. However, analysis of subgroups showed
that mortality was significantly improved with infusion for
patients admitted in Killip Class 1 status (relative risk
0.28) and for patients with diabetes (relative risk 0.31).
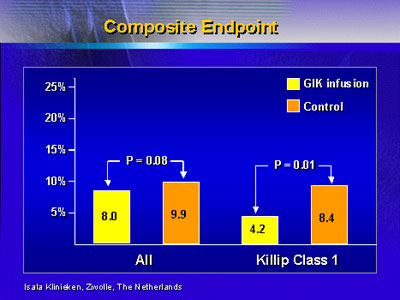
Composite endpoint was also improved with infusion for patients
in Killip Class 1.
Data on infarct size according to serial enzyme levels was
available for 622 of the 940 patients, and analysis showed
that patients in the highest quartile of enzyme peak level
were more likely to have an anterior infarction, be admitted
in Killip Class 2, 3, or 4, and to have no TIMI 3 blood flow
after angioplasty.
The risk for post-angioplasty ejection fraction of less
than 30 percent was higher for patients who were older, had
an anterior infarction, had high enzyme peak levels, and who
were diabetic.
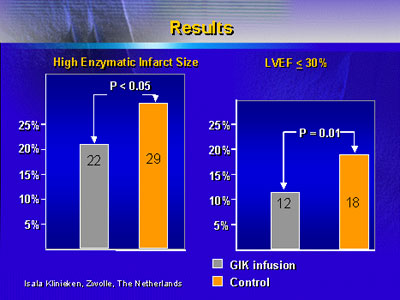
The primary conclusion of the study was the significant
reduction in 30-day mortality and composite endpoint for patients
admitted in Killip Class 1. Additional findings were an improvement
with infusion in infarct size based on blood enzyme levels
and in post-angioplasty left ventricular ejection fraction.
Although there seems to be a benefit of combination infusion
therapy, especially for patients in Killip Class 1, conclusions
are limited by lack of data for all patients. For instance,
data on enzyme levels were incomplete (and thus omitted from
analysis) for 318 patients, often due to in-hospital death
or early transfer to another institution.
|