Phase
I trial results show that autologous skeletal myoblast transplantation
is a feasible treatment of postinfarction myocardial injury.
The improvements in left ventricular ejection fraction investigators
saw at 4 months persisted at a 12 month evaluation. This treatment
warrants further studies.
The primary problems in the setting of post-myocardial infarction
heart failure is the loss of myocardium and replacement of
with fibrous scar tissue. Recent advances in biotechnology
have allowed investigators to consider restoring missing contractile
elements within the left ventricle. They hope to improve contractile
function in patients with post-myocardial infarction heart
failure.
Investigators have considered several types of cells for
possible use to improve contractile dysfunction. These include
stem cells, cardiomyocytes and fibroblasts. Dr. Siminiak and
colleagues have studied the possibility of using autologous
skeletal myoblasts for replacing missing myocardium.
There are several advantages to using autologous cells for
this application. Patients will not need immunosuppression.
Supply of cells is not a problem. Furthermore, the use of
autologous cells does not raise the ethical issues that have
surrounded the potential use of embryonic or fetal cells.
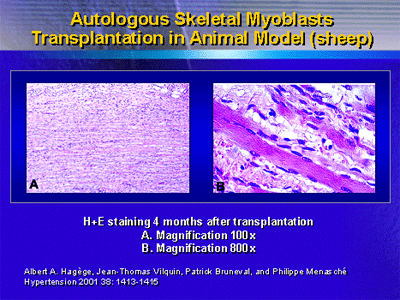
Preclinical data show that transplanted skeletal myoblasts
in the post-infarction scar form myocyte-like elements that
possibly restore function. Investigators have replicated these
findings in a number of species. Hagège et al. showed
the effects of transplanting autologous skeletal myoblasts
in sheep.
Dr. Siminiak and colleagues launched an independent phase
I clinical trial to evaluate the safety and feasibility of
transplanting skeletal myoblasts during coronary artery bypass
graft surgery. This investigation included 10 patients about
to undergo surgery. All patients had akinesia or dyskinesia
involving 1 to 3 segments of the left ventricle.
For each patient, researchers obtained a cell sample from
the vastus lateralus approximately 1 cubic centimeter in size.
They isolated the myoblasts and put them in cell culture for
3 weeks. They injected the resulting myoblasts into the akinetic
or dyskinetic area during the bypass procedure.
One patient died 7 days after the procedure. However, the
autopsy revealed a recent myocardial infarction in an area
that was previously normokinetic. They assumed this new infarction
was not related to the transplantation of cells and continued
the trial.
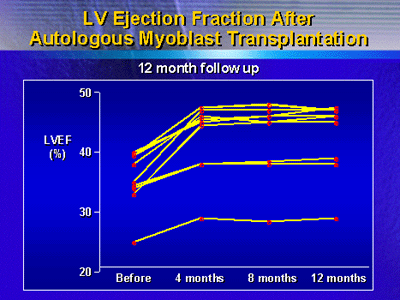
Previously, investigators reported an increase in ejection
fraction for all 9 surviving participants. Here at ACC, Dr.
Siminiak showed that this increase in ejection fraction persisted
throughout the 12 month follow-up period.
Investigators also observed changes in segmental contractility
at 5 months. Dr. Siminiak said 5 of 9 dyskinetic segments
became akinetic, and 4 of 10 akinetic segments became hypokinetic.
After observing episodes of serious ventricular tachycardia
in the first 2 patients, the researchers decided to use prophylactic
amiodarone. In the following patients, they observed no sustained
ventricular tachycardia episodes. Only 2 patient were on oral
amiodarone at 2 months. No patient remained on amiodarone
at 3 months.
These results suggest autologous skeletal myoblast transplantation
is at least feasible. However, many questions remain. Investigators
must conduct further studies to validate this method and establish
its potential role in clinical practice. For example, a phase
II study will provide data on left ventricular performance
improvement after transplantation.
|