This
selective aldosterone blocker reduced total mortality by 15%
in patients with acute myocardial infarction and early complications
of heart failure. Incidence of cardiovascular deaths and hospitalizations
was also significantly lower versus placebo. All patients were
on optimal medical therapy. These results suggest eplerenone
may reduce the number of deaths and hospitalizations above what
clinicians can achieve with current standard treatments alone.
In post-myocardial infarction patients who
also have left ventricular dysfunction and heart failure,
ACE inhibitors and beta blockade are the therapies of choice.
While treatment is effective, these patients still experience
considerable morbidity and mortality.
Some recent efforts have failed to achieve
additional improvements in these patients. However, one approach
with promise is the use of aldosterone blocking agents. Recent
research shows that aldosterone blockade can decrease mortality
and morbidity in patients who have severe chronic heart failure
from left ventricular dysfunction. One aldosterone blocker
under study is spironolactone. In the Randomized Aldactone
Evaluation Study (RALES), spironolactone reduced mortality
by 30% in heart failure patients who were taking ACE inhibitors.
Another potentially useful agent is eplerenone,
a selective aldosterone blocker that is approved in the United
States for treating hypertension. Dr. Pitt and colleagues
hypothesized that eplerenone would reduce both mortality and
morbidity in post-myocardial infarction patients with left
ventricular dysfunction and heart failure.
To test this, they enrolled more than 6,600
patients with acute myocardial infarction, left ventricular
ejection fraction less than or equal to 40%, and documented
heart failure. Patients underwent randomization to eplerenone
or placebo in addition to optimal medical therapy. The eplerenone
dose was 25 mg per day, titrated upward to a maximum of 50
mg per day. In addition, all patients received optimal medical
therapy; this could include ACE inhibitors, beta blockers,
diuretics, angiotensin-receptor blockers and coronary reperfusion
therapy.
One primary endpoints of the study was all
cause mortality. Dr. Pitt reported that there were significantly
fewer deaths in the eplerenone group. Over a mean 16 months
of follow-up, investigators recorded 478 deaths in that group,
compared with 554 deaths in the placebo group, with a relative
risk of 0.85 (95% CI 0.75 - 0.96; p=0.008).
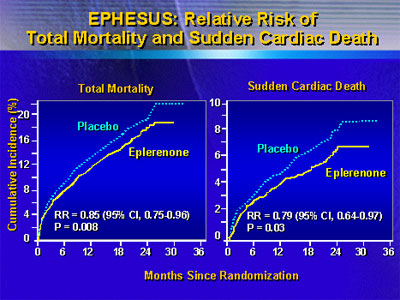
Another primary endpoint was the composite
of cardiovascular death and hospitalization for acute myocardial
infarction, heart failure, stroke or arrhythmia. Again, there
were fewer cardiovascular deaths or hospitalizations in the
eplerenone group, with a relative risk of 0.87 (95% CI 0.79
- 0.95; p=0.002).
|
EPHESUS: Primary Endpoints
| |
Placebo
(n=3,313) |
Eplerenone
(n=3,319) |
Relative Risk
(95% CI) |
p
value |
| Death from any cause |
554 |
478 |
0.85
(0.75-0.96) |
0.008 |
| Cardiovascular deaths
and hospitalizations |
993
|
885 |
0.87
(0.79-0.95) |
0.002 |
Source: Pitt et al., N Engl J Med
2003; 348: 1309-21.
|
Eplerenone was also superior to placebo in
a variety of secondary endpoints, including death from any
cause or any hospitalization (8% reduction, p=0.02), sudden
cardiac death (21% reduction, p=0.03) and episodes of hospitalization
from heart failure (23%, p=0.02).
Investigators reported that hyperkalemia was
a side effect of eplerenone treatment. They saw a 5.5% rate
of serious hyperkalemia, versus 3.9% for placebo (p=0.002).
The rate of hypokalemia was 8.4% percent for the eplerenone
group, compared with 13.1% percent for placebo (p<0.001).
These findings suggest adding eplerenone to
optimal medical treatment can decrease morbidity and mortality
in acute myocardial infarction patients who have left ventricular
dysfunction and heart failure. They strongly support the suggestion
that many post-myocardial infarction patients should be taking
an aldosterone receptor blocker in addition to beta blockers
and ACE inhibitors.
While this was not a comparative trial, Dr.
Pitt said he believed eplerenone and spironolactone would
provide a similar degree of aldosterone blockade. However,
there would likely be a difference in side effect profile.
Spironolactone treatment can result in impotence, gynecomastia
and breast pain, among other side effects. In the EPHESUS
study, investigators reported no excess of gynecomastia or
impotence. Because of this difference, eplerenone may have
the potential to extend the benefits of aldosterone blockade
to a much wider group of patients, Dr. Pitt said.
|