The clinical
benefit of a paclitaxel-eluting coronary stent appears to extend
over a prolonged period of time. This suggests the drug-eluting
stent does not delay major cardiovascular events, but actually
prevents them. The study results also support the safety of
discontinuing antiplatelet treatment 6 months after stent implantation.
The TAXUS II trial compared different formulations of the
TAXUS stent, which releases the restenosis-inhibiting drug
paclitaxel over a period of time. Investigators presented
the 6 month results of the TAXUS II trial at a previous medical
conference. Results showed that in coronary arteries, both
formulations of the stent efficiently and safely reduced in-stent
neointimal formation, binary restenosis and the need to perform
repeat revascularizations.
The objective of the 12-month follow-up analysis is to evaluate
continued safety and efficacy of the stent 6 months after
discontinuation of antiplatement treatment with clopidogrel.
Dr. Colombo reported the 12-month clinical follow-up of the
TAXUS II trial here at an ACC late breaking clinical trial
session.
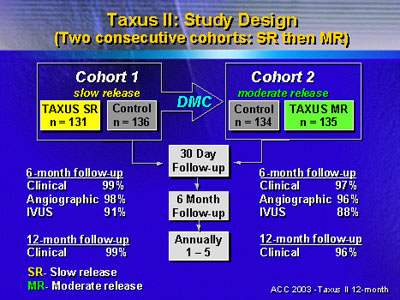
The randomized trial included more than 500 patients randomized
to TAXUS SR (slow release), TAXUS MR (moderate release) or
a control arm that received a bare metal stent.
The data at 6 months showed that the cumulative incidence
of major adverse coronary events is higher among patients
who did not receive the drug-eluting stent. Events occurred
in 19.8% (52 of 263) patients in the bare metal stent group,
but only 8.5% (11 of 130) patients who received TAXUS SR and
7.8% (10 of 129) patients who received TAXUS MR.
The overall benefit of the drug-eluting stent continued
at 12 months, Dr. Colombo said. Cumulative event rates are
now 21.7% (57 of 263) for the control group, 10.9% (14 of
129) for TAXUS SR, and 9.8% (13/132) for TAXUS MR.
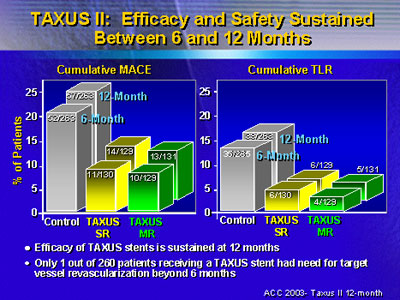
Similar data emerged when Dr. Colombo compared incidence
of target vessel revascularization at 6 and 12 months. Target
vessel revascularization at 6 months was higher in the bare
metal stent group: 13.2% (35 of 265) patients versus 4.6%
(6 of 130) and 3.1% (4 of 129) in the TAXUS SR and MR groups,
respectively.
Again, the benefit of the drug-eluting stent continued at
12 months. Only 1 of 260 patients receiving a TAXUS stent
needed target vessel revascularization beyond 6 months after
implantation, compared with 3 additional patients in the control
group.
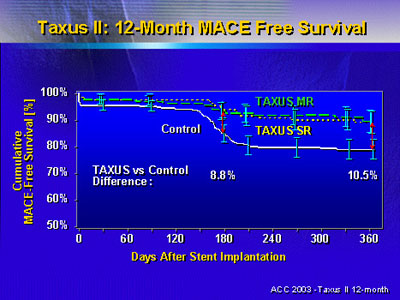
Survival free of major adverse coronary events at 6 months
in the patients that received TAXUS stents was 8.8 percentage
points higher than patients who received the bare metal stent.
At 12 months, the difference in survival free of events was
10.5 percentage points. Dr. Colombo said these data suggest
TAXUS stents do not delay in-stent restenosis, but actually
prevent its occurrence.
|