Interim
results of this trial show that in patients treated for in-stent
restenosis, a particular type of beta radiation delivery catheter
system reduces target vessel revascularization at 9 months by
42%. Investigators also reported a very low in-stent restenosis
rate. These results confirm that vascular brachytherapy is useful,
particularly for the treatment of in-stent restenosis.
The only technology proven to be effective for treating
in-stent restenosis is vascular brachytherapy. While this
success is encouraging, there are a number of opportunities
to improve outcomes. These include optimizing the dose of
radiation and improving the design and position of the radiation
source. Studies that address these concerns have the potential
to improve outcome.
One study to address these potential improvements is BRITE
II (Beta Radiation to Reduce In-Stent
Restenosis). Investigators designed this study to test
the feasibility, efficacy and safety of the RDX system, which
incorporates a new balloon-shaped source design to deliver
beta radiation.
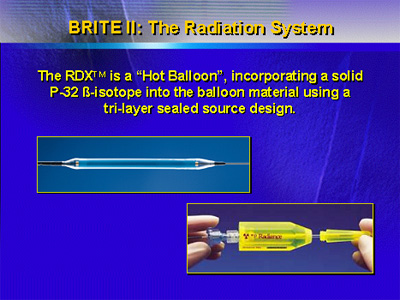
The RDX system incorporates the beta-emitting P-32 beta-isotope
into the balloon material with a three-layer, sealed source
design. Investigators wanted to know if this particular system
would produce an improved reduction in restenosis and revascularization
rates.
The primary endpoint of the BRITE II trial is target vessel
revascularization at 9 months. Investigators defined target
vessel revascularization as any clinical revascularization
of the target vessel using percutaneous coronary intervention
or coronary artery bypass graft. Investigators also looked
at a variety of safety and angiographic endpoints, including
binary restenosis rate and late thrombosis or total occlusion.
The study included 460 patients (mean age 62 years) with
in-stent restenosis. All patients received treatment with
angioplasty and stenting. They were randomized in a 3-to-1
fashion to beta radiation delivery (360 patients) or placebo
(120 patients). The dose of radiation was 20 Gy at 1 mm from
the source.
Twenty-six U.S. centers contributed patients to the study.
All patients had a single restenotic lesion in a stent in
a native coronary vessel. The degree of target vessel occlusion
had to be greater than 50% but could not be total occlusion.
Total lesion length was less than 45 mm.
Dr. Waksman presented the preliminary analysis of BRITE
II data. The analysis suggested the treatment was safe. This
was reflected in a procedural success rate exceeding 95%,
a less than 1% rate of periprocedural complications, and a
low incidence of 30-day major adverse clinical events (27%
versus 43% for placebo, p=0.02).
|
BRITE II: Clinical Outcomes
to 9 Months
| |
P-32 |
Placebo |
*p
value |
Δ% |
| N
(follow up) |
302
(94%) |
89
(84%) |
|
|
| |
|
|
|
|
| |
|
|
|
|
| Death |
1% |
2% |
NS |
|
| Any
MACE |
27% |
45% |
0.02 |
↓Δ
40% |
* Interim
Results
|
The final analysis for efficacy is not complete. However,
investigators did meet the study assumption that there would
be a 42% reduction in target vessel revascularization. Target
vessel revascularization at 9 months was 25% versus 43% (42%
reduction, p=0.02). This compares favorably and is perhaps
superior to historical data on the effect of vascular brachytherapy.
In 3 different trials, vascular brachytherapy reduce target
vessel revascularization versus placebo by about one-third.
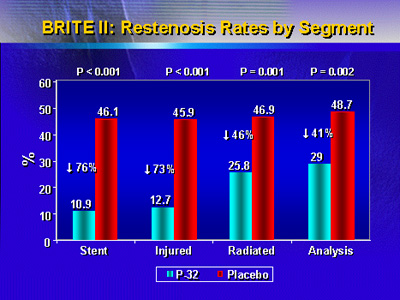
In addition, there was a very low rate of in-stent restenosis
in patients who received the beta radiation compared with
placebo (10.9% versus 46.1%, p<0.001). This represents a 76%
reduction in in-stent restenosis versus placebo.
Due to commercial considerations, the RDX system will probably
not be released for marketing and clinical use. However, these
findings confirm that vascular brachytherapy is useful and
effective in the treatment of in-stent restenosis.
The company Endologix manufactured the RDX system and sponsored
the BRITE II study. Investigators will present 12-month clinical
follow-up data at a later time.
|