| Investigators
compared data on heart failure patients in carvedilol clinical trials
with patients who received carvedilol in community medical practice.
Patients in the community were older, more often female, and had less
severe heart failure than patients in clinical trials. There were
differences in titration rates and target dosage. However, most patients
in COHERE continued on treatment. The results suggest that community
physicians can successfully initiate and maintain carvedilol treatment.
Results of heart failure trials may not be generalizable to community
practice. This is partly because patients in clinical trials are
different. They are usually younger and more often male. They have
less comorbidity and are more likely to adhere to medication regimens,
compared with patients in the community.
Another factor is the treating physician. In clinical trials, doctors
are more specialized. They are more likely to have support from
nurses and coordinators compared with community practitioners.
A registry that illustrates experience with carvedilol in community
treated heart failure patients is now available. This registry is
called the Coreg Heart Failure Registry (COHERE). It includes data
from 633 participating physicians on 4,273 unselected heart failure
patients.
Physicians who contributed data to the COHERE registry include
cardiologists and non-cardiologists who work in the community practice
setting. The data set does not include the experience of full time
faculty members or specialists in heart failure.
To illustrate differences between clinical trials and community
practice, investigators compared COHERE registry with data from
1,094 patients in carvedilol randomized clinical trials and 2,981
patients in open label carvedilol compassionate use trials.
Investigators have not yet fully evaluated outcomes data. However,
some interesting differences emerged.
The COHERE registry contained more older patients and more women
than in the clinical trials. In addition, patients in COHERE had
higher left ventricular ejection fraction and less severe New York
Heart Association functional class than the clinical trial patients.
Patient Characteristics: COHERE and Carvedilol Clinical Trials
| |
COHERE
|
Compassionate use
|
Clinical trials
|
| Age (years) |
66 ± 13
|
60 ± 13
|
58 ± 12
|
| Female |
35%
|
25%
|
23%
|
| New York Heart
Association class III/IV |
38%
|
62%
|
47%
|
| Mean left ventricular
ejection fraction (LVEF) |
31 ± 12%
|
Not available
|
23 ± 7%
|
All comparisons of compassionate use or clinical trials vs. COHERE
were significant (P < 0.001).
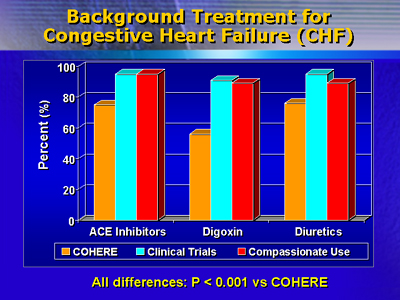
The COHERE patients were also less likely to be on background treatment
with digoxin, diuretics and angiotensin converting enzyme inhibitors.
Titration took longer in the COHERE registry patients, and fewer
patients reached target maintenance doses of 25-50 mg twice daily
compared with clinical trial patients. On the other hand, most patients
continued carvedilol treatment in COHERE.
Titration Experience
|
|
Carvedilol titration experience
|
COHERE titration experience
|
|
|
COHERE
(n=3,861)
|
Clinical
trials
|
Compassionate use
|
Cardiologists'
patients
(n = 2,869)
|
Non-cardiologists'
patients
(n = 992)
|
|
No. titration days
|
75 ± 46
|
NA
|
54 ± 32*
|
75 ± 45
|
75 ± 48
|
|
Titrated to 25-50 mg bid
|
45%
|
85%*
|
93%*
|
50%
|
30%**
|
|
Carvedilol discontinued
|
9%
|
12%
|
7%*
|
10%
|
6%**
|
* P < 0.001 vs. COHERE
** P < 0.001
NA = Not available
As expected, physicians participating in COHERE were less likely
to be cardiologists. They were also less to have an academic affiliation
or practice at a hospital.
This registry data provides a broader picture of physician practice
in the community. Future observational studies should also consider
the differences between clinical trials and community experience
in the treatment of heart failure with beta-blockers.
|