| Analysis
of CarvedilOl ProspEctive RaNdomiIzed CUmulative Survival (COPERNICUS)
data suggests that pretreatment blood pressure is not a determinant
of the efficacy or safety of carvedilol in patients with severe heart
failure. Because prior heart failure survival trials with the beta-blockers
metoprolol and bisoprolol excluded patients with systolic blood pressures
<100 mm Hg, physicians may be reluctant to prescribe beta-blockers
(especially those with a vasodilatory effect, like carvedilol) in
patients with low systolic pressure.
In COPERNICUS, 2,289 patients with symptoms of heart failure at
rest or on minimal exertion were randomized to placebo or carvedilol.
Patients had left ventricular ejection fraction <25%, systolic
blood pressure 385 mm Hg, and were receiving diuretics, and angiotensin
converting enzymes with or without digoxin. The trial was terminated
prematurely on account of a marked 35% survival benefit with carvedilol.
In the COPERNICUS population, 132 (5.8%) had systolic blood pressure
of 85-95 mm Hg and 264 (11.5%) had a systolic blood pressure of
96-105 mm Hg at randomization. Patients with lower systolic blood
pressures (<96 mm Hg) tended to be younger and to have lower
ejection fractions. Patients with lower systolic blood pressure
had significantly worse prognoses (P < 0.0001 for all cause mortality)
than those with higher blood pressures (>125 mm Hg), with about
a 50% mortality rate at 2 years. Survival was best in those patients
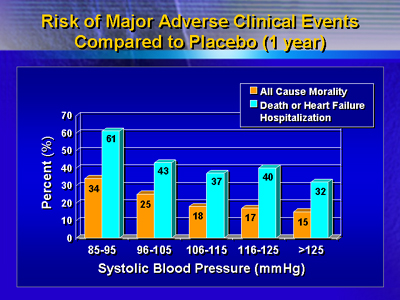
with systolic blood pressure >125 mm Hg. Death or hospitalization
for any reason, similarly, occurred in nearly 80% of patients in
the lowest (<96 mm Hg) systolic blood pressure group at one year,
and was less common (P < 0.0001) progressively with higher systolic
blood pressures.
Carvedilol reduced risk of death or risk of death or heart failure
hospitalization among all subgroups defined by baseline systolic
blood pressure. The magnitude of benefit was independent of systolic
blood pressure (interaction P = 0.64 and P = 0.80 for mortality
and for death or heart failure hospitalization, respectively). Risk
of worsening heart failure increased with lower systolic blood pressure
levels in the placebo group. That risk was reduced similarly (interaction
P = 0.74) across groups by the addition of carvedilol, indicating
that the benefit is entirely independent of blood pressure.
Risk of Outcome Variables (Hazard Ratio) Carvedilol: Placebo
| Systolic blood pressure |
All cause mortality |
Death or heart failure hospitalization |
| 85-95 mm Hg |
0.77
|
0.74
|
| 96-105 mm Hg |
0.61
|
0.75
|
| 106-115 mm Hg |
0.65
|
0.78
|
| 116-125 mm Hg |
0.61
|
0.54
|
| >125 mm Hg |
0.60
|
0.68
|
The rate of permanent withdrawal decreased with increasing baseline
systolic blood pressure similarly for both placebo and carvedilol.
While hypotension was reported more frequently with carvedilol than
with placebo, the effect was not accentuated at lower systolic blood
pressures.
Dr. Rouleau concluded that pretreatment blood pressure is not a
determinant of efficacy or safety of carvedilol in patients with
severe heart failure, and thus should not be used to exclude patients
from treatment with the drug as long as systolic blood pressure
is 385 mm Hg. Also, the vasodilatory effects of carvedilol did not
adversely affect patients with a low systolic blood pressure and
may have contributed to the reduction in risk of worsening heart
failure produced by treatment.
|