| This
is the first prospective trial showing statin therapy reduces major
adverse cardiac events in patient who previously underwent a percutaneous
coronary intervention. Fluvastatin reduced risk of events by 22% over
4 years. This finding supports the use of lipid lowering therapy in
this patient population.
Percutaneous coronary interventions are successful in the short-term.
However, patients who undergo these procedures are still at risk
for subsequent cardiovascular events. An average of 40% of patients
undergoing percutaneous coronary intervention will have a myocardial
infarction, or require repeat intervention or bypass within 5 years.
The Lescol Intervention Prevention Study (LIPS) is the first prospective,
double blind, placebo controlled study of statins in this population
of patients who have undergone angioplasty and other interventional
procedures.
Investigators from 57 centers in 10 countries enrolled a total
of 1,677 patients (mean age 60 years, about 84% male) into the LIPS
study. All patients had average cholesterol levels.
Patients received fluvastatin 40 mg twice daily or placebo after
a first successful percutaneous coronary intervention. Follow-up
was at least 3 years per patient. The primary endpoint was time
to first major adverse coronary event, including cardiac death,
nonfatal myocardial infarction, bypass or repeat percutaneous coronary
intervention.
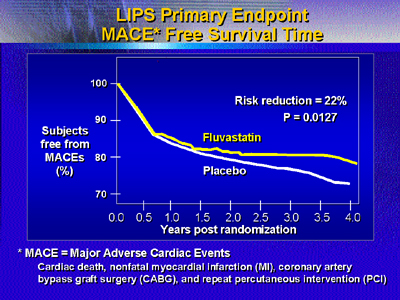
Fluvastatin treatment reduced the risk of major adverse cardiac
events (MACE) 22% compared with placebo (P = 0.013). Risk ratios
for all primary and secondary endpoints favored fluvastatin treatment.
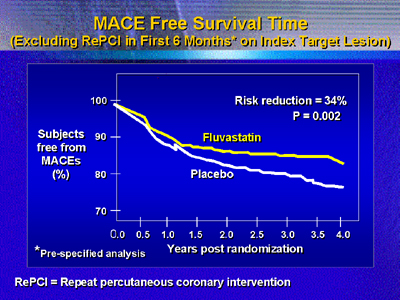
In a prespecified analysis that excluded repeat intervention, risk
reduction was 34% compared with placebo. Patients with diabetes,
which comprised 12% of the study population, had a 47% reduction
in risk of a serious cardiac event (P = 0.041). Patients with multivessel
disease, or 37% of the study population, had a 34% reduction vs.
placebo (P = 0.011).
Safety analysis suggested no significant elevations of creatine
phosphokinase. A recent pooled analysis of 9,000 patients on fluvastatin
found the rate of clinically relevant elevations in creatine phosphokinase
was not significantly different than in patients on placebo.
The LIPS study demonstrates the value of starting lipid lowering
therapy in patients who undergo cardiac interventions. The results
also validate aggressive treatment of high risk patients with diabetes
or multivessel disease.
Importantly, the findings support the recommendation to reduce
low density lipoprotein cholesterol below 100 mg/dL in all patients
who have had a percutaneous coronary intervention. This recommendation
is part of the most recent guidelines from the National Cholesterol
Education Program.
|