| Protagonist:
The Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events
(CURE) trial was the largest ever study of acute coronary syndromes.
It clearly showed the benefits of clopidogrel in acute coronary syndromes
in a wide variety of patients. By contrast, trials of platelet glycoprotein
IIb/IIIa antagonists show very inconsistent effects. Not every patient
with acute coronary syndrome patient will benefit from treatment with
one of these agents.
Antagonist: Available evidence provides
a strong rationale for using clopidogrel early in a wide range of
patients with acute coronary syndromes. However, platelet glycoprotein
IIb/IIIa antagonists should be used in certain patients who present
to the emergency department. This includes troponin positive patients,
and anyone whose management will include an invasive strategy.
Protagonist: The CURE trial was a randomized, double blind,
parallel group trial of acute and long-term clopidogrel therapy
vs. placebo. It included 12,562 patients with acute coronary syndromes.
Investigators randomized patients to an immediate 300 mg clopidogrel
loading dose or placebo, then 75 mg clopidogrel or placebo for up
to 1 year.
The primary endpoint of CURE was a composite of myocardial infarction,
stroke and cardiovascular death 1 year after randomization. Trial
results showed a highly significant 20% relative risk reduction
in this endpoint (P = 0.00009).
CURE Main Efficacy Results
| |
Clopidogrel / aspirin |
Placebo / aspirin |
Relative risk reduction |
| Myocardial infarction |
5.2%
|
6.7%
|
23%
|
| (ST elevation
myocardial infarction) |
1.9%
|
3.1%
|
40%
|
| Stroke |
1.2%
|
1.4%
|
14%
|
| Cardiovascular
death |
5.1%
|
5.5%
|
7%
|
| Primary endpoint (combined
myocardial infarction, stroke, cardiovascular death) |
5.1%
|
5.5%
|
7%*
|
*P = 0.00009
Statistical analysis showed that the benefit was already apparent
as early as 2 hours after the loading dose. This correlated nicely
with basic science data showing a rapid rise in platelet inhibition
following administration of a loading dose.
Patients benefited regardless of whether they underwent percutaneous
coronary intervention or medical therapy. In patients who underwent
stenting, there was a 44% relative risk reduction in the primary
endpoint.
This effect was also evident across low, medium and high risk patient
groups. Absolute risk reduction in patients with low and moderate
risk score patients was 1.6%. Absolute risk reduction in high risk
patients was almost 5%.
There was a consistent benefit regardless of other standard therapies
the patient received, including heparin, aspirin, angiotensin converting
enzyme inhibitors, angioplasty, bypass and others.
Results of the major IIb/IIIa antagonist trials are very different.
Clearly, these agents benefit patients who undergo intervention.
One meta-analysis of 6 interventional trials including 14,706 patients
shows a remarkable reduction in death or myocardial infarction at
30 days (8.7% to 5.6%).
However, IIb/IIIa antagonists have shown little efficacy in general
trials of acute coronary syndromes. A meta-analysis including approximately
31,000 patients shows only a 9% relative risk reduction.
Furthermore, this effect is not consistent. For example, there is
no benefit in patients over 70 years of age. There is a significant
15% excess in death and myocardial infarction in women. That starkly
contrasts with the CURE trial of clopidogrel. In more than 50 subgroups
examined, every one echoed the significant risk reduction.
There is also a significant 62% excess of bleeding with IIb/IIIa
antagonists. On the other hand, the excess bleeding in CURE was
lower than in all IIb/IIIa trials individually. Therefore, a strategy
of starting this agent in case the patient needs percutaneous coronary
intervention later may actually be causing net harm.
Antagonist: Clopidogrel and IIb/IIIa inhibitors are both
very effective treatments. To maximize patient outcomes, clinicians
should use each agent when appropriate.
Published guidelines now state that acute coronary syndrome patients
undergoing percutaneous coronary intervention should receive a IIb/IIIa
inhibitor. A dramatically compelling and consistent benefit can
be seen across various interventional trials including IIb/IIIa
inhibitors. Reductions in death and myocardial infarction are in
the range of 50% to 70%.
The IIb/IIIa inhibitors and clopidogrel have not been compared
directly in the setting of acute coronary syndromes. However, from
review of the available data, it seems reasonable that IIb/IIIa
inhibitors would provide a benefit greater than clopidogrel in percutaneous
coronary intervention.
However, IIb/IIIa inhibitors are not for everyone. The relative
benefit of this approach increases in higher risk patients. Probably
the best risk stratifier is troponin status. Many investigators
believe troponins particularly suited for identifying patients who
will have a major benefit.
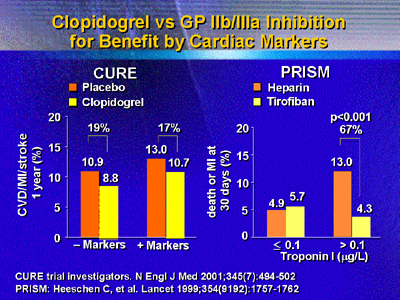
Data from the Platelet Receptor Inhibition for Ischemic Syndrome
(PRISM) trial showed a 70% reduction in death or myocardial infarction
at 30 days for troponin positive patients. Interestingly, this benefit
occurred with or without revascularization. In contrast, clopidogrel
showed no such relationship in a study of positive and negative
markers.
The IIb/IIIa approach also has merit in patients with diabetes.
Antiplatelet therapy has a dramatic effect in treating acute coronary
syndromes in this rapidly growing population. One recent meta-analysis
suggested an approximately 25% reduction in mortality. In the largest
clopidogrel study ever, there was a 7% reduction in mortality that
did not reach statistical significance.
Some of the most recent data supporting use of IIb/IIIa inhibitors
in interventional procedures comes from the Treat Angina with Aggrastat
and Determine Cost of Therapy with an Invasive or Conservative Strategy
(TACTICS) study.
In this study, investigators evaluated effectiveness in clinical
practice of IIb/IIIa inhibitor added to background therapy in an
invasive vs. conservative strategy. They saw an early and significant
reduction in myocardial infarction. This suggests the benefit of
IIb/IIIa seen in clinical trials is also applicable in clinical
practice.
|