| The
landmark Randomized Evaluation of Mechanical Assistance for Treatment
of Congestive Heart Failure (REMATCH) trial showed a 46% reduction
in 2-year mortality vs. medical therapy in patients with end stage
heart failure. This new analysis of the data shows that the major
benefit of mechanical assistance is in patients sick enough to require
intravenous inotropic therapy at baseline. There does not appear to
be a benefit in patients who were less compromised at baseline.
The REMATCH trial was an evaluation of mechanical cardiac support
devices for end stage heart failure patients not eligible for transplant.
Left ventricular assist devices have been very successful as bridges
to cardiac transplantation. In REMATCH, investigators evaluated
mortality over 2 years in patients with very advanced heart failure
who received permanent implantation of the HeartMate (Thoratec Corp.).
This intervention has been called "destination therapy."
The hypothesis of REMATCH was that this left ventricular assist
device would reduce 2- year mortality by 33% vs. optimal medical
management. The landmark results of REMATCH instead showed a larger
benefit of a 46% reduction in mortality (2-year mortality of 28%
vs. 10%).
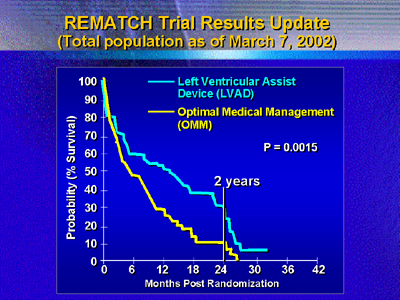
Investigators must now determine which patients are most likely
to benefit from mechanical assistance devices. In this recent analysis,
they hypothesize that patients sick enough to require inotropic
therapy would have the major survival benefit and meaningful quality
of life improvements.
Investigators chose to look at the impact of inotropic therapy
because they were used surprisingly frequently. At the time of randomization,
an unexpectedly high number of patients (71%, or 91 out of 129)
were on intravenous inotropic infusions.
These patients on intravenous inotropic support at baseline had
clinical profiles worse than the other REMATCH subjects. This was
illustrated by lower systolic blood pressure, more pronouncedly
dysregulated sodium levels and higher intracardiac pressures.
For patients who received optimal medical management, there was
a clear trend toward worse survival among patients on inotropic
infusions at baseline (P = 0.11).
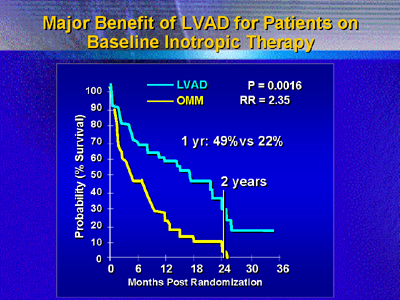
The benefit of the left ventricular assistance device was high
in patients on inotropic drugs at baseline. Survival at 6 months
was 58% for those who received the device, vs. 39% for those on
medical therapy. By 1 year, survival was over twice as high (49%
vs. 22%, P = 0.0016). By 2 years, survival was 24% for patients
with devices, and all the medical therapy patients had died.
That obvious survival benefit contrasts with no significant difference
in survival for device vs. medical therapy for in the patients not
on inotropic therapy at baseline (P = 0.52). It does not appear
that a less compromised population would have significantly improved
survival on the device.
Quality of life analysis suggests favorable and meaningful improvements
for all survivors among the REMATCH subjects on inotropic drugs
at baseline. Improvements in domains of physical and emotional function
were greater at 1 year vs. the few survivors on medical therapy.
The National Heart, Lung and Blood Institute and Thoratec Corp.
jointly sponsored the REMATCH trial.
|