| Thrombin
inhibitors were developed once the crystalline structure of thrombin
was characterized. Thrombin inhibitors may bind to the active site,
fibrinogen exosite and/or the heparin-binding site. 2 classes of thrombin
antagonists have been developed: bivalent antagonists and monovalent
or univalent antagonists. Bivalent antagonists may be associated with
bleeding problems while univalent compounds have fewer acute ischemic
events and a reduction in bleeding complications. Univalent compounds
are safer than heparin.
Thrombin plays a role in the generation of the thrombus and coagulation.
It is the most potent biological activator of platelets and is involved
in cell migration, activation of the vascular epithelium and in
stabilization of the clot.
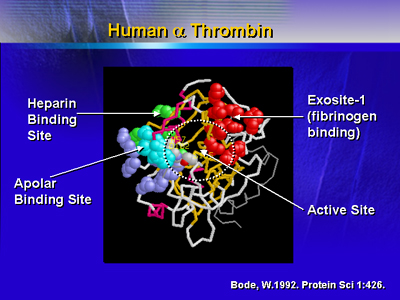
Thrombin inhibitors were developed after the crystalline structure
of thrombin was identified. Thrombin inhibitors act at particular
sites on the thrombin molecule. These sites are the active site,
fibrinogen exosite and the heparin-binding site. The active site
is located in a pocket within the molecule. This site is responsible
for most of the proteolytic activity. The fibrinogen binding exosite
is responsible for platelet activation. It is also the site where
substrates are tethered so they can be cleaved by the active site.
The heparin-binding site is the place where heparin binds to the
thrombin molecule.
Heparin is often used as an anticoagulant. Heparin has several
limitations including variable bioavailability, increases in affinity
of thrombin for fibrin, and stimulation of platelet aggregation.
Because of these limitations, alternative drugs such as thrombin
antagonists were developed.
2 classes of thrombin antagonists are available: bivalent antagonists
and monovalent or univalent antagonists. Univalent antagonists are
small molecules that sit in the active site and block the access
of substrates to this site. Argatroban is a typical drug in this
class.
Bivalent antagonists are larger molecules whose N-terminus sits
over the active site and the carboxyterminus fits over the exosite.
The bivalent antagonist prevents access to both of these sites.
Herudin is the prototypic bivalent antagonist. It is the most potent
and binds with the highest affinity.
The advantages of thrombin antagonists are predictable bioavailability,
predictable anti-coagulant responses, no known pro-coagulation effects,
no rebound, and more effective and consistent penetration of the
thrombus.
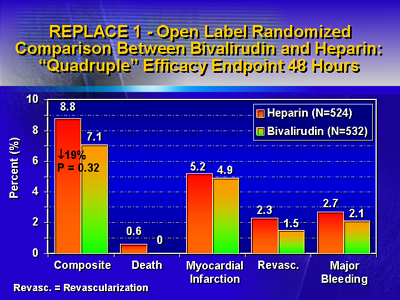
Clinical trials have reported safety concerns with herudin and
include major bleeding and a narrow therapeutic window. In contrast,
univalent compounds are associated with fewer acute ischemic events
and a reduction in bleeding complications. As a result, Dr. Kleiman
concluded univalent compounds are safer than heparin. Combination
thrombolytic therapy using a fibrinolytic with a thrombin antagonist
has not proven effective, and is not recommended.
|