|
This study included patients admitted with
unstable angina or acute myocardial infarction. Short-term treatment
with azithromycin did not reduce death or occurrence of ischemic
events. In addition, the subgroup of patients without antibodies
to Chlamydia pneumoniae did not have a treatment benefit.
The rate of recurrent ischemic events is high in the 6 months following
an initial acute coronary syndrome. The cause for recurrent ischemic
events is vascular inflammation leading to disruption of plaque
and thrombosis. This may be related to infection of the vessel wall
with Chlamydia pneumoniae.
Initial studies found azithromycin or roxithromycin treatment reduced
inflammation of the vasculature and recurrence of ischemic events.
Therefore, treatment with azithromycin, initiated after presentation
with acute coronary syndromes, may reduce the occurrence of recurrent
ischemic events. This was the hypothesis of the Azithromycin on
Recurrent Ischemic Events in Patients with Acute Coronary Syndrome
(AZACS) trial.
The AZACS trial was a randomized, double blind, placebo controlled
evaluation of patients admitted with acute myocardial infarction
or unstable angina. A total of 7 centers participated in the trial.
Investigators randomized 1,439 patients to azithromycin or placebo.
The azithromycin dose was 500 mg for 1 day, then 250 mg per day
in the following 4 days.
Patients were followed for 6 months. The primary endpoint was all
cause mortality, nonfatal myocardial infarction, or recurrent ischemia
that required bypass or percutaneous coronary intervention.
Patient characteristics were similar for azithromycin and placebo
groups. Mean age was 64 and 65 years, respectively. Approximately
one quarter of patients were women.
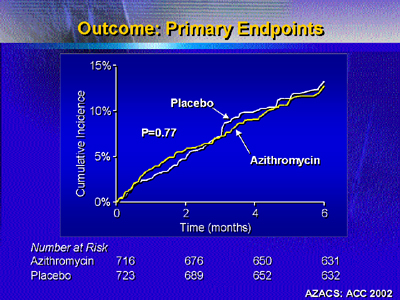
Results showed no statistically significant difference in primary
endpoints between the 2 groups. Incidence of any primary event was
14.3% for placebo and 14.9% for azithromycin. Incidence of individual
endpoints (death, nonfatal myocardial infarction, revascularization)
was not different.
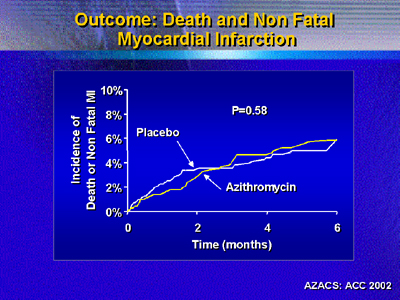
Investigators prespecified an analysis of data excluding events
related to the qualifying event. This was because early events may
dilute the potential positive effect of azithromycin later in the
trial.
However, this analysis also showed no difference in the composite
primary endpoint (12.6% for placebo vs. 12.3% for azithromycin).
Again, there were no significant differences in incidence of the
specific endpoints of death, nonfatal myocardial infarction and
revascularization.
| |
Patients (%)
|
|
Placebo
|
Azithromycin
|
| Primary endpoints |
|
|
Death
|
3.5
|
3.1
|
Nonfatal myocardial infarction
|
2.4
|
2.1
|
Revascularization
|
6.8
|
7.1
|
| Secondary
endpoints |
|
|
Unstable angina/
congestive heart failure
|
19.9
|
20.1
|
Similarly, there was no difference between azithromycin and placebo
for the secondary endpoint of unstable angina/congestive heart failure.
The other prespecified subgroup was the 80% of patients with positive
Chlamydia pneumoniae antibody titers. Again, however, investigators
found no difference between azithromycin and placebo.
It remains unclear whether there is any role for antibiotic treatment
in acute coronary syndromes. However, results of AZACS should be
evaluated in context of other studies evaluating different agents
and protocols.
Investigators received support from The Heart Fund at Cedars-Sinai
and from institutional funds of participating centers.
|