| This
trial compared a heparin-coated stent, an uncoated stent and balloon
angioplasty in the treatment of stenosis in small, native coronary
arteries. The stent groups showed a marginally significant benefit
in minimal lumen diameter vs. balloon angioplasty. The heparin-coated
stent did not provide angiographic or clinical benefit over the uncoated
stent in these small vessels.
It is unclear whether stent implantation or balloon angioplasty
is superior for treating symptomatic coronary artery stenosis in
smaller vessels. In addition, there is no conclusive data illustrating
the role of heparin-coated stents in these smaller vessel lesions.
The objective of the Heparin-Coated Stents in Small Coronary Arteries
Trial (COAST) was to assess angiographic and clinical benefit of
a heparin-coated stent (Jostent Flex) in the stenoses of small native
coronary arteries.
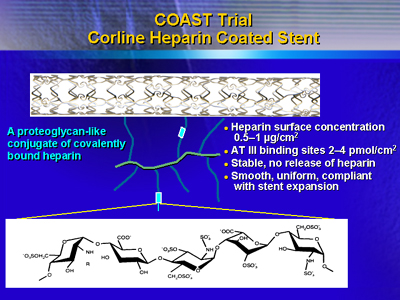
A total of 21 centers throughout Europe participated in the trial.
Investigators randomized 605 patients with angina to one of three
arms: balloon angioplasty, uncoated Jostent Flex stent, or heparin-coated
Jostent Flex stent. Pretreatment included aspirin and 10,000 IU
Heparin.
The target lesion vessel diameter had to be between 2.0 and 2.6
mm. The primary endpoint was minimal lumen diameter at 6 months.
There were no significant differences in baseline patient characteristics
or lesion distribution. In each study arm, about 80% of patients
had multi-vessel disease. Angiographic results showed a near perfect
match in reference diameter among the three groups (2.31 to 2.33
mm).
After intervention, there was a larger minimal lumen diameter,
greater acute gain, and less residual stenosis in the stent groups.
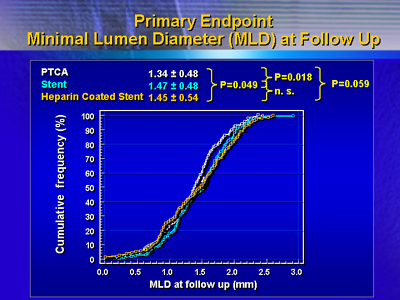
At 6 months, there was a marginally significant benefit in minimal
lumen diameter at 6 months favoring stent treatment. Minimal lumen
diameter was 1.34 mm for balloon angioplasty, 1.47 mm for the heparin-coated
stent, and 1.45 for the uncoated stent (P = 0.018).
A net gain difference favored the stent groups. However, late loss
was identical in the stent groups and angioplasty group.
These results did not translate into a reduced rate of restenosis:
32% for balloon angioplasty, 25% for the uncoated stent, and 30%
for the heparin-coated stent.
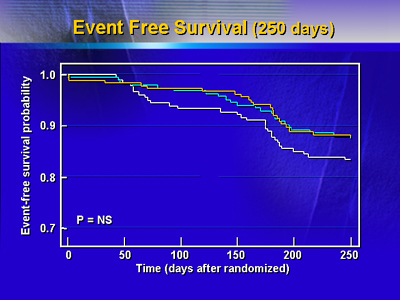
There were no statistically significant differences in number serious
adverse events at 250 days. There were two deaths in both stent
arms and none in the balloon angioplasty arm. Target vessel revascularization
was somewhat higher in the balloon angioplasty arm. Event free survival
at 250 days was 88% in both stent arms and 84% in the balloon angioplasty
arm.
Taken together, these results suggest a borderline benefit in minimal
lumen diameter in favor of stenting. Furthermore, there was no angiographic
or clinical benefit of the heparin-coated Jostent Flex stent over
the uncoated stent.
|